Last updated April 28, 2023

COVID-19 is still a global pandemic. Around the world, as of 5:40pm CEST, 26 April 2023, there have been 764,474,387 cumulative confirmed cases of COVID-19, including 6,915,655 deaths, reported to the World Health Organization. As of 24 April 2023, a total of 13,325,228,015 vaccine doses have been administered. The adoption of vaccines worldwide continues to increase, yet periodic spikes and surges in infection rates continue to occur with new SARS-CoV-2 variants, such as that observed in Australia in Jan 2022. Vaccine booster doses provide effective protection against developing severe disease and hospitalization, but vaccine adoption and distribution face ongoing challenges in low- and middle-income (LMIC) countries (1). The development of intranasal vaccines could help alleviate some of the challenges in these areas. Therapeutic interventions for those already infected are in development, with one (Paxlovid) currently available under emergency use authorization (EUA) in the US.
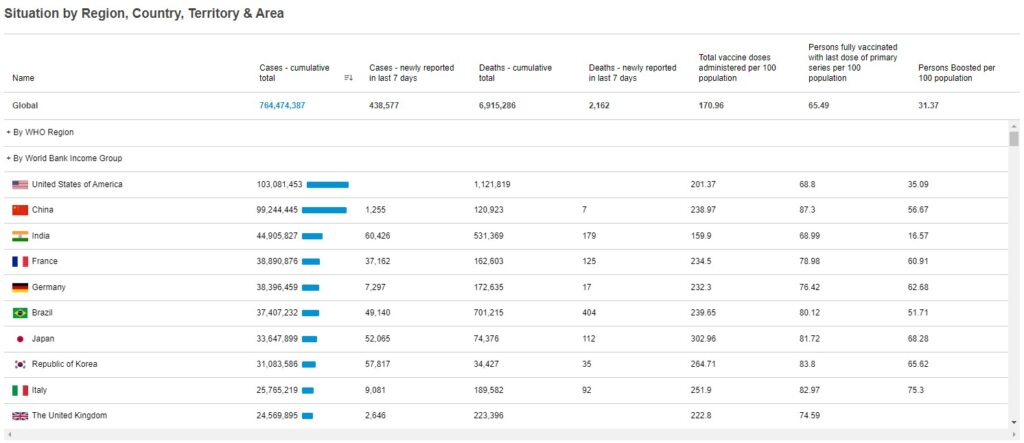
Cumulative COVID-19 statistics by country: WHO COVID-19 Dashboard. Geneva: World Health Organization, 2020. Available online: https://covid19.who.int/ (last cited: April 28, 2023).
Continue reading “COVID-19 Intranasal Vaccines: Right on the Nose?”