Last updated April 28, 2023

COVID-19 is still a global pandemic. Around the world, as of 5:40pm CEST, 26 April 2023, there have been 764,474,387 cumulative confirmed cases of COVID-19, including 6,915,655 deaths, reported to the World Health Organization. As of 24 April 2023, a total of 13,325,228,015 vaccine doses have been administered. The adoption of vaccines worldwide continues to increase, yet periodic spikes and surges in infection rates continue to occur with new SARS-CoV-2 variants, such as that observed in Australia in Jan 2022. Vaccine booster doses provide effective protection against developing severe disease and hospitalization, but vaccine adoption and distribution face ongoing challenges in low- and middle-income (LMIC) countries (1). The development of intranasal vaccines could help alleviate some of the challenges in these areas. Therapeutic interventions for those already infected are in development, with one (Paxlovid) currently available under emergency use authorization (EUA) in the US.
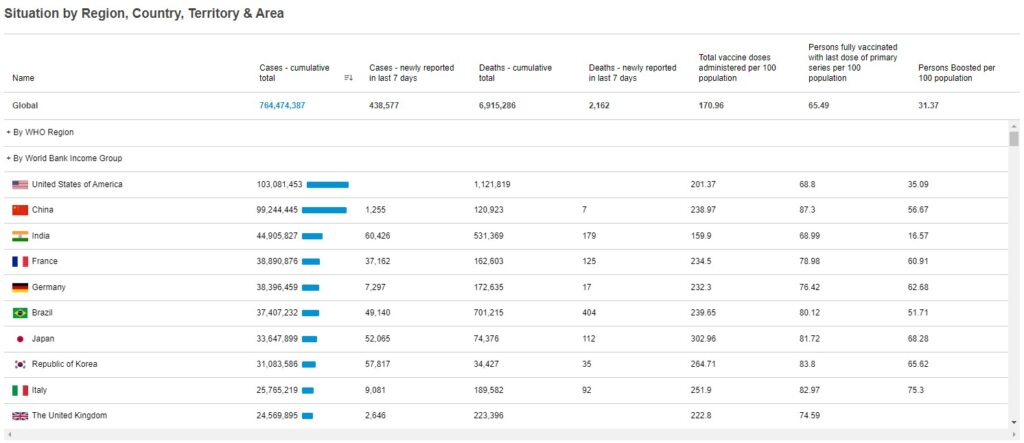
Cumulative COVID-19 statistics by country: WHO COVID-19 Dashboard. Geneva: World Health Organization, 2020. Available online: https://covid19.who.int/ (last cited: April 28, 2023).
Current COVID-19 vaccines, although diverse in composition (mRNA, adenoviral vector-based and protein subunits) have one feature in common: the route of administration. They are all administered by intramuscular injection. Intramuscular vaccines generate a systemic immune response, producing antibodies against the virus that circulate through the blood to other parts of the body. While these intramuscular vaccines have proven effective against developing severe symptoms of COVID-19, they do not necessarily stop viral infection at the source—the mucous membranes of the upper respiratory tract—as discussed previously. A systemic immune response is mediated primarily by immunoglobulin G (IgG), while mucosal immunity is mediated primarily by IgA, and protection of the nasal passages happens only at high titers of IgG (2).
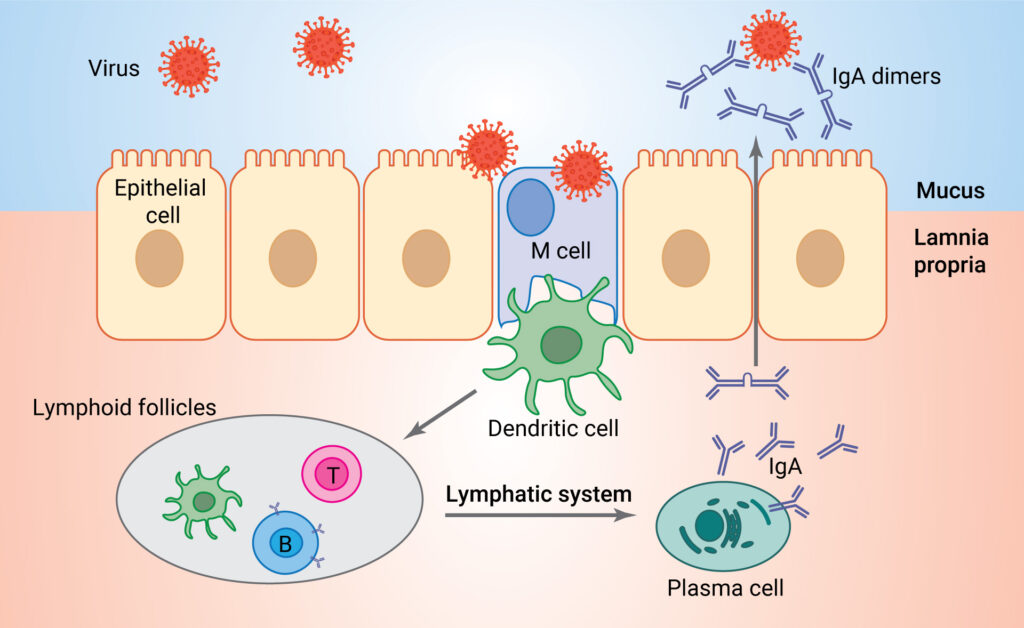
It seems logical to develop vaccine strategies that treat the infection at the source, repelling invaders at the gates before they have a chance to run amok within a city. Although early COVID-19 vaccine development efforts did include intranasal vaccines, they were in the minority, and relatively little attention has been paid to intranasal vaccine strategies in the mainstream media—until recently.
Media coverage by Scientific American, PBS News Hour and the New York Times, among others, has driven a public interest in COVID-19 intranasal therapies These articles focused on results from preclinical (animal model) trials of several intranasal vaccine candidates. In the fall of 2022, two nasal vaccinations were approved for use in India and China, and recently, Nouailles and colleagues described a live- attenuated vaccine for SARS-CoV-2 that has shown promise in hamsters. As of Jan 2023, there are eleven intranasal vaccines in clinical trials according to the WHO vaccine tracker.
Challenges and Opportunities
Intranasal vaccines, while showing significant promise, also face some challenges. While the route of administration is easier than intramuscular injections, making them particularly attractive for young children (like the FluMist influenza vaccine), ensuring the correct dose is administered can be difficult (Mouro, 2022). For example, the recipient could sneeze out part of the vaccine, resulting in a lower effective dose than administered. Further, the thick mucus layer in the upper respiratory tract may hinder the absorption of vaccine components. The IgA response in the mucosa is typically short-lived, so repeated doses of the intranasal vaccine may be required to provide effective protection. Interestingly, a novel approach to overcoming some of these problems may lie in the use of nanomaterials as delivery vehicles (reviewed in 8, 9).
Despite these challenges, the advantages of intranasal vaccines—lower production, storage and distribution costs—coupled with their noninvasive route of administration make them an attractive option to prevent COVID-19 infection at the source. A combination of current intramuscular vaccines followed by intranasal boosters may prove to be the best defense against SARS-CoV-2 infection.
Learn about tools to study SARS-CoV-2 with our SARS-CoV-2 Drug Discovery and Vaccine Development page.
References
- Mobarak, A.M. et al. (2022) End COVID-19 in low- and middle-income countries. Science 375(6585), 1105.
- Lund, F.E. and Randall, T.D. (2021) Scent of a vaccine: Intranasal vaccination should block SARS-CoV-2 transmission at the source. Science 373(6553), 397–399.
- Flitter, B.A. et al. (2022) [preprint] Mucosal immunization of cynomolgus macaques with adenoviral vector vaccine elicits neutralizing nasal and serum antibody to several SARS-CoV-2 variants. bioRxiv doi: https://doi.org/10.1101/2022.02.21.481345
- Lam, J.-Y. et al (2022) A nasal omicron vaccine booster elicits potent neutralizing antibody response against emerging SARS-CoV-2 variants. Emerg. Microbes & Infect. 11(1), 964–967.
- Lei, H. et al. (2022) Intranasal administration of a recombinant RBD vaccine induces long-term immunity against Omicron-included SARS-CoV-2 variants. Signal Transduct. Target Ther. 7, 159.
- Mao, T. et al. (2022) [preprint] Unadjuvanted intranasal spike vaccine booster elicits robust protective mucosal immunity against sarbecoviruses. bioRxiv doi: https://doi.org/10.1101/2022.01.24.477597
- Mouro, V. and Fischer, A. (2022) Dealing with a mucosal viral pandemic: lessons from COVID-19 vaccines. Mucosal Immunol. doi: https://doi.org/10.1038/s41385-022-00517-8
- Wilson, B. and Geetha, K.M. (2022) Nanomedicine to deliver biological macromolecules for treating COVID-19. Vaccine doi: https://doi.org/10.1016/j.vaccine.2022.05.068
- Tang, J. et al. (2022) Nanotechnologies in delivery of DNA and mRNA vaccines to the nasal and pulmonary mucosa. Nanomaterials 12, 226.
