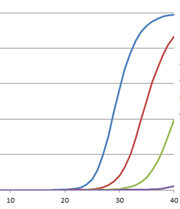
We have all been hearing a lot about RT-PCR, rRT-PCR and RT-qPCR lately, and for good reason. Real-Time Reverse Transcriptase Polymerase Chain Reaction (rRT-PCR) is the technique used in by the Center for Disease Control (CDC) to test for COVID-19. Real-time RT-PCR, or quantitative RT-PCR (RT-qPCR)*, is a specialized PCR technique that visualizes the amplification of the target sequence as it happens (in real-time) and allows you to measure the amount of starting target material in your reaction. You can read more about the basics of this technique, and watch a webinar here. For more about RT-PCR for COVID-19 testing, read this blog.
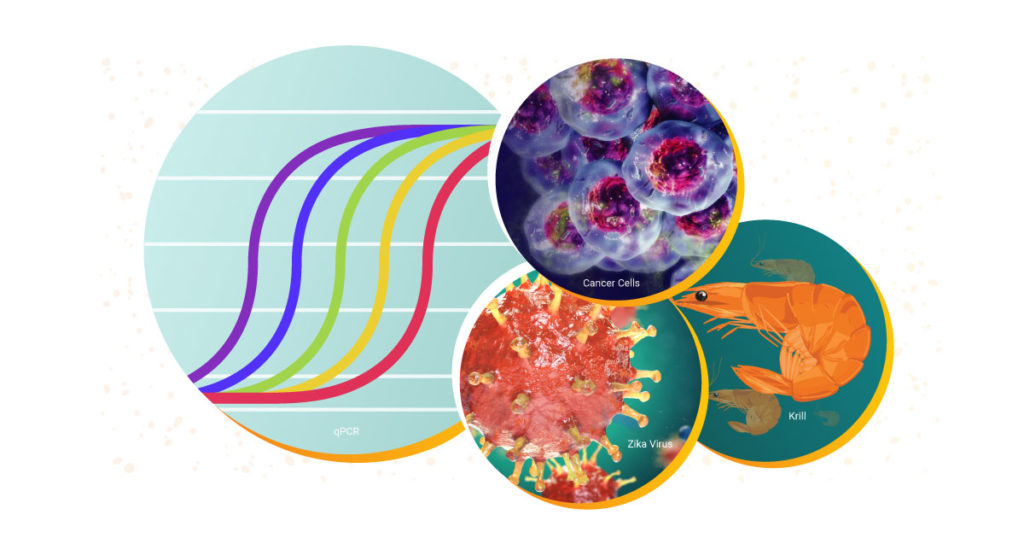
Both qPCR and RT-qPCR are powerful tools for scientists to have at their disposal. These fundamental techniques are used to study biological processes in a wide range of areas. Over the decades, Promega has supported researchers with RT-qPCR and qPCR reagents and systems to study everything from diseases like COVID-19 and cancer to viruses in elephants and the circadian rhythm of krill.
Continue reading “RT-qPCR and qPCR Assays—Detecting Viruses and Beyond”