At the time of writing this post, no scientist had yet discovered the secret to immortality. In our world, we’ve come to accept that living things are born, grow old and die—the circle of life.
And yet, for many years, life scientists believed that the circle of life did not apply to our constituent cells when cultured in a laboratory. That is, cultured normal human cells were immortal, and they would continue to grow and proliferate forever, as long as they were provided with the necessary nutrients.
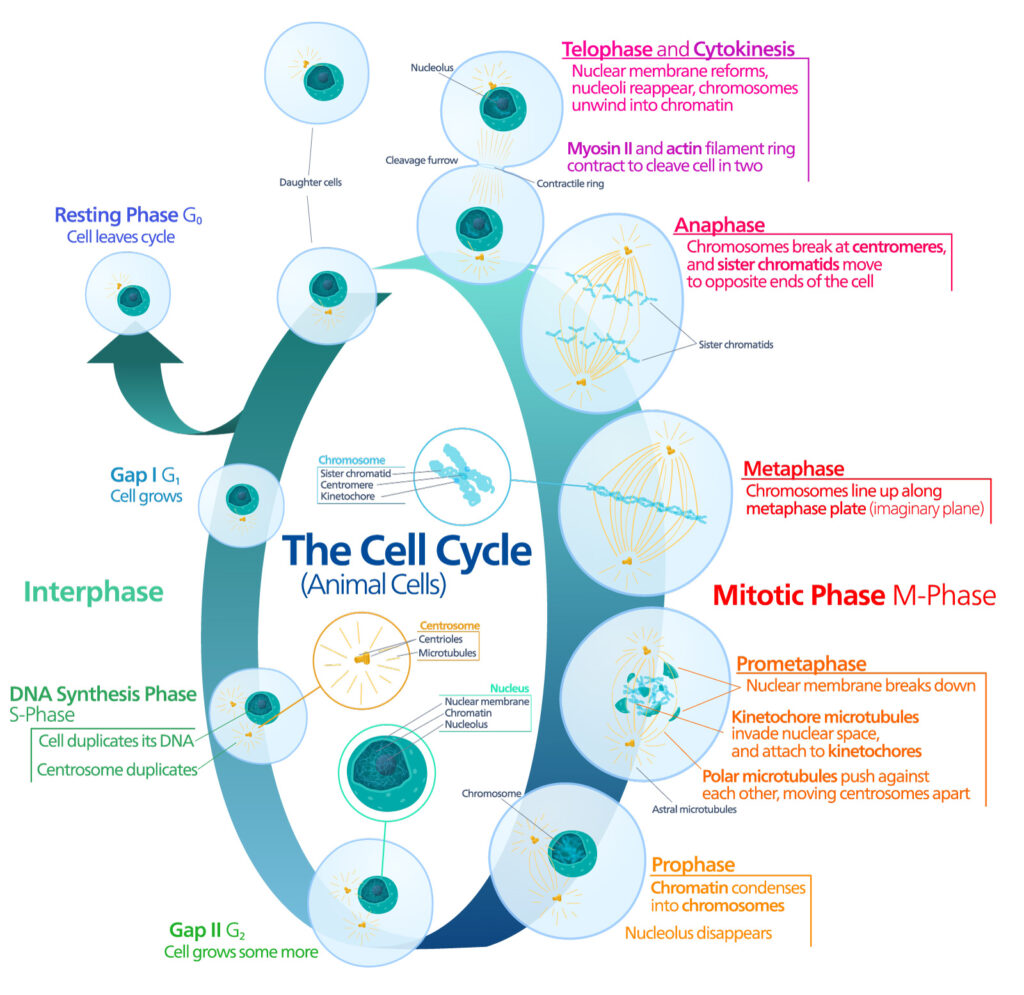
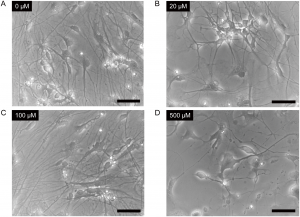
Pioneering work published in 1961 by Leonard Hayflick and Paul Moorhead challenged that theory (reviewed in 1). Their research showed that normal cells in culture have a finite capacity to replicate. After they reach a certain number of replicative cycles, cells stop dividing. Hayflick and Moorhead made the important distinction between normal human cells and cultured cancer cells, which are truly immortal. In later years, the limit to the number of replicative cycles normal human cells can undergo became known as the Hayflick limit. Although some scientists still express skepticism about these findings, the Hayflick limit is widely recognized as a fundamental principle of cell biology.
Continue reading “Cellular Senescence and Cancer Therapy: Overcoming Immortality?”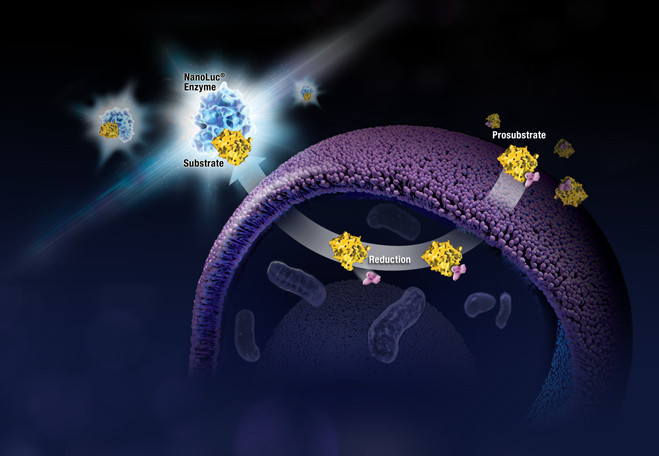

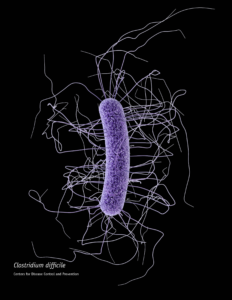 When someone is admitted to a hospital for an illness, the hope is that medical care and treatment will help them them feel better. However, nosocomial infections—infections acquired in a health-care setting—are becoming more prevalent and are associated with an increased mortality rate worldwide. This is largely due to the misuse of antibiotics, allowing some bacteria to become resistant. Furthermore, when an antibiotic wipes out the “good” bacteria that comprise the human microbiome, it leaves a patient vulnerable to opportunistic infections that take advantage of disruptions to the gut microbiota.
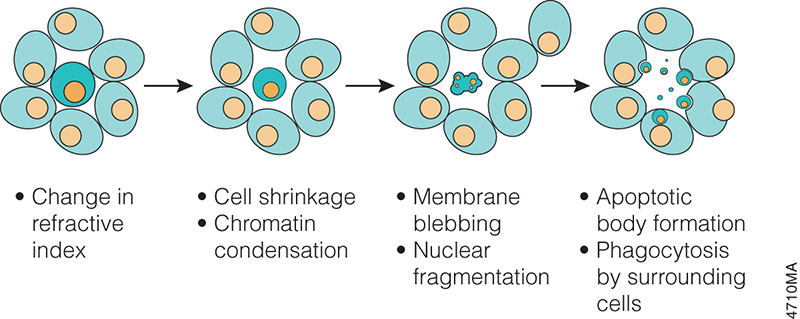
When someone is admitted to a hospital for an illness, the hope is that medical care and treatment will help them them feel better. However, nosocomial infections—infections acquired in a health-care setting—are becoming more prevalent and are associated with an increased mortality rate worldwide. This is largely due to the misuse of antibiotics, allowing some bacteria to become resistant. Furthermore, when an antibiotic wipes out the “good” bacteria that comprise the human microbiome, it leaves a patient vulnerable to opportunistic infections that take advantage of disruptions to the gut microbiota.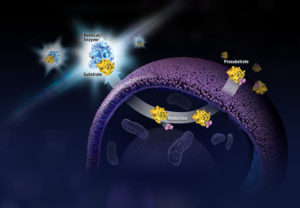 What if you could uncover a small but significant cellular response as your population of cells move toward apoptosis or necrosis? What if you could view the full picture of cellular changes rather than a single snapshot at one point? You can! There are real-time assays that can look at the kinetics of changes in cell viability, apoptosis, necrosis and cytotoxicity—all in a plate-based format. Seeking more information? Multiplex a real-time assay with endpoint analysis. From molecular profiling to complementary assays (e.g., an endpoint cell viability assay paired with a real-time apoptosis assay), you can discover more information hidden in the same cells during the same experiment.
What if you could uncover a small but significant cellular response as your population of cells move toward apoptosis or necrosis? What if you could view the full picture of cellular changes rather than a single snapshot at one point? You can! There are real-time assays that can look at the kinetics of changes in cell viability, apoptosis, necrosis and cytotoxicity—all in a plate-based format. Seeking more information? Multiplex a real-time assay with endpoint analysis. From molecular profiling to complementary assays (e.g., an endpoint cell viability assay paired with a real-time apoptosis assay), you can discover more information hidden in the same cells during the same experiment.