At the time of writing this post, no scientist had yet discovered the secret to immortality. In our world, we’ve come to accept that living things are born, grow old and die—the circle of life.
And yet, for many years, life scientists believed that the circle of life did not apply to our constituent cells when cultured in a laboratory. That is, cultured normal human cells were immortal, and they would continue to grow and proliferate forever, as long as they were provided with the necessary nutrients.
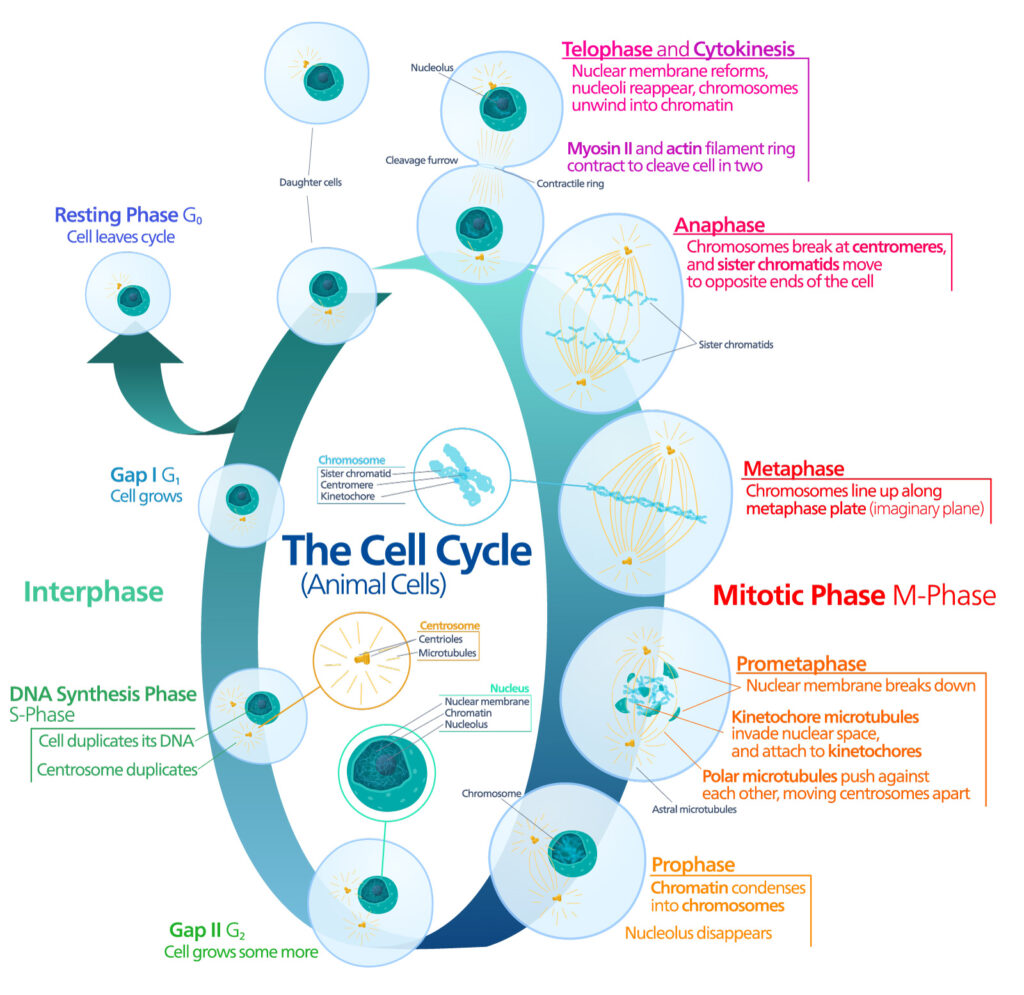
Pioneering work published in 1961 by Leonard Hayflick and Paul Moorhead challenged that theory (reviewed in 1). Their research showed that normal cells in culture have a finite capacity to replicate. After they reach a certain number of replicative cycles, cells stop dividing. Hayflick and Moorhead made the important distinction between normal human cells and cultured cancer cells, which are truly immortal. In later years, the limit to the number of replicative cycles normal human cells can undergo became known as the Hayflick limit. Although some scientists still express skepticism about these findings, the Hayflick limit is widely recognized as a fundamental principle of cell biology.
What happens to cells once they reach the Hayflick limit? Typically, they enter a state of cellular senescence, where cell growth is arrested, and the cells stop dividing. This phenomenon isn’t confined to cultured cells in a laboratory—it happens within living organisms as well. Although it can be part of a normal developmental process, senescence is usually triggered as a response to stress factors. Common examples include DNA damage caused by exposure to radiation or toxic chemicals, tissue injury or metabolic disturbances (2). Senescence can also be induced by oncogenic insults. Since senescent cells can no longer proliferate, this response can act as a powerful tumor-suppressive mechanism (3).
The end of the line for normal cells is programmed cell death or apoptosis. This process is tightly controlled and initiated by either intrinsic or extrinsic pathways. Caspases play central roles in apoptosis, ultimately leading to protein degradation and cell death. However, senescent cells often exhibit a characteristic resistance to apoptosis (4).
Senescence: The Good, the Bad, and the Ugly
Senescent cells exhibit distinct morphological features and significant changes in gene expression that distinguish them from proliferating cells (4). At the protein level, these complex changes result in the secretion of inflammatory cytokines, chemokines, growth factors and other signaling molecules in a characteristic signature known as the senescence-associated secretory phenotype (SASP).
At first glance, inducing cell senescence would seem to provide an ideal therapeutic pathway for treating cancer, because cell senescence shuts down the uncontrolled proliferation associated with tumor development. Many anticancer drugs, as well as radiation, are associated with induction of senescence in both tumor and normal tissues, i.e., therapy-induced senescence or TIS (5).
However, TIS is a double-edged sword. Some components of the SASP are linked to the promotion of tumor development (5). TIS can also cause chronic inflammation and resistance to chemotherapeutic agents (3). Ultimately, due to the genomic instability of cancer cells, they can escape the growth arrest of senescence and return to uncontrolled proliferation, leading to cancer relapse (5).
Resolving this dilemma led to the emergence of a new class of “senolytic” drugs: small-molecule compounds that selectively target senescent tumor cells for destruction, typically by inducing apoptosis (6). This mode of therapy could serve as an adjuvant to traditional chemotherapy or radiation therapy for cancer treatment.
Following the TRAIL to Improve Cancer Therapy
One promising candidate for cancer therapy is the tumor necrosis factor-related apoptosis inducing ligand (TRAIL). This transmembrane protein induces extrinsic apoptosis by binding to death receptors DR4 and DR5. TRAIL also binds to two membrane decoy receptors, DcR1 and DcR2, that are unable to activate apoptotic signaling (7). A study by two groups at the University of Groningen, Netherlands aimed to determine the sensitivity of senescent cancer cells to TRAIL (8).
Using TRAIL-sensitive breast cancer and ovarian cancer cell lines, the researchers induced senescence by treatment with the anticancer drug doxorubicin. They monitored senescence by qRT-PCR with panels of senescence-associated and TRAIL receptor genes, after isolating RNA using the Maxwell® LEV simplyRNA Cells/Tissue Kits. Cells were treated with recombinant human TRAIL and cell viability was measured. The researchers observed variable TRAIL sensitivity among the cell lines tested. Their data suggested that increased resistance to extrinsic apoptosis was not a common feature of TIS cancer cells.
Next, the researchers measured apoptotic activity using the Caspase-Glo® 3/7 Assay and monitored TRAIL signaling through expression levels of various TRAIL receptors. These experiments suggested that, despite retaining apoptotic abilities and expressing higher levels of DR5, some TIS cancer cells might acquire resistance to TRAIL-induced apoptosis by overexpressing various decoy receptors.
Based on observing strong upregulation of DR5 but not DR4 in TRAIL-resistant senescent cells, the researchers examined whether a DR5-selective TRAIL variant could increase the apoptotic response. Their data showed increased activity of the DR5-selective TRAIL variant (DHER), compared to wild type TRAIL, in eliciting caspase-dependent apoptosis in senescent cancer cells, with no obvious toxicities for normal cells. To confirm these results, the researchers used a senescent 3D spheroid model. Measuring 3D spheroid viability using the CellTiter-Glo® 3D Assay, caspase activity using the Caspase-Glo® 3/7 3D Assay, and cytotoxicity using the CellTox™ Green Cytotoxicity Assay demonstrated the effectiveness of TRAIL DHER in eliminating TIS cancer cells.
The authors conclude that their results demonstrate the acquired vulnerability of TIS cancer cells and suggest the use of selective DR5 stimulation may become a viable strategy for improving cancer therapies. Further research into similar pathways to induce apoptosis in TIS cancer cells is progressing on many fronts. Increasing our understanding of cellular senescence in cancer and the development of new therapies specifically targeting senescent tumor cells could, in time, deliver the death blow to many types of cancer.
Explore the full range of Promega cell health assays to measure cell viability, cytotoxicity, apoptosis and necrosis.
References
- Shay, J.W. and Wright, W.E. (2000) Hayflick, his limit, and cellular ageing. Nat. Rev. 1, 72–76.
- Gorgoulis, V. et al. (2019) Cellular senescence: Defining a path forward. Cell 179, 813–827.
- Demaria, M. et al. (2017) Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 7(2) 165–176.
- Kumari, R. and Jat, P. (2021) Mechanisms of cellular senescence: Cell cycle arrest and senescence associated secretory phenotype. Front. Cell Dev. Biol. 9, 645593.
- Wang, B. et al. (2020) Senescent cells in cancer therapy: Friends or foes? Trends Cancer 6(10), 838–857.
- Carpenter, V. et al. (2021) Senolytics for cancer therapy: Is all that glitters really gold? Cancers 13, 723.
- Thorburn, A. (2007) Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) pathway signaling. J. Thorac. Oncol. 2, 461–465.
- Soto-Gamez, A. et al. (2022) Enhanced extrinsic apoptosis of therapy-induced senescent cancer cells using a death receptor 5 (DR5) selective agonist. Cancer Lett. 525, 67–75.
Latest posts by Ken Doyle (see all)
- Will Artificial Intelligence (AI) Transform the Future of Life Science Research? - February 1, 2024
- RAF Inhibitors: Quantifying Drug-Target Occupancy at Active RAS-RAF Complexes in Live Cells - September 5, 2023
- Synthetic Biology: Minimal Cell, Maximal Opportunity - July 25, 2023

In Hardvard University some ways, a living cell is like a shoreline, where some creatures build their homes on rocky, solid structures while others live in shifting and dynamic sands.