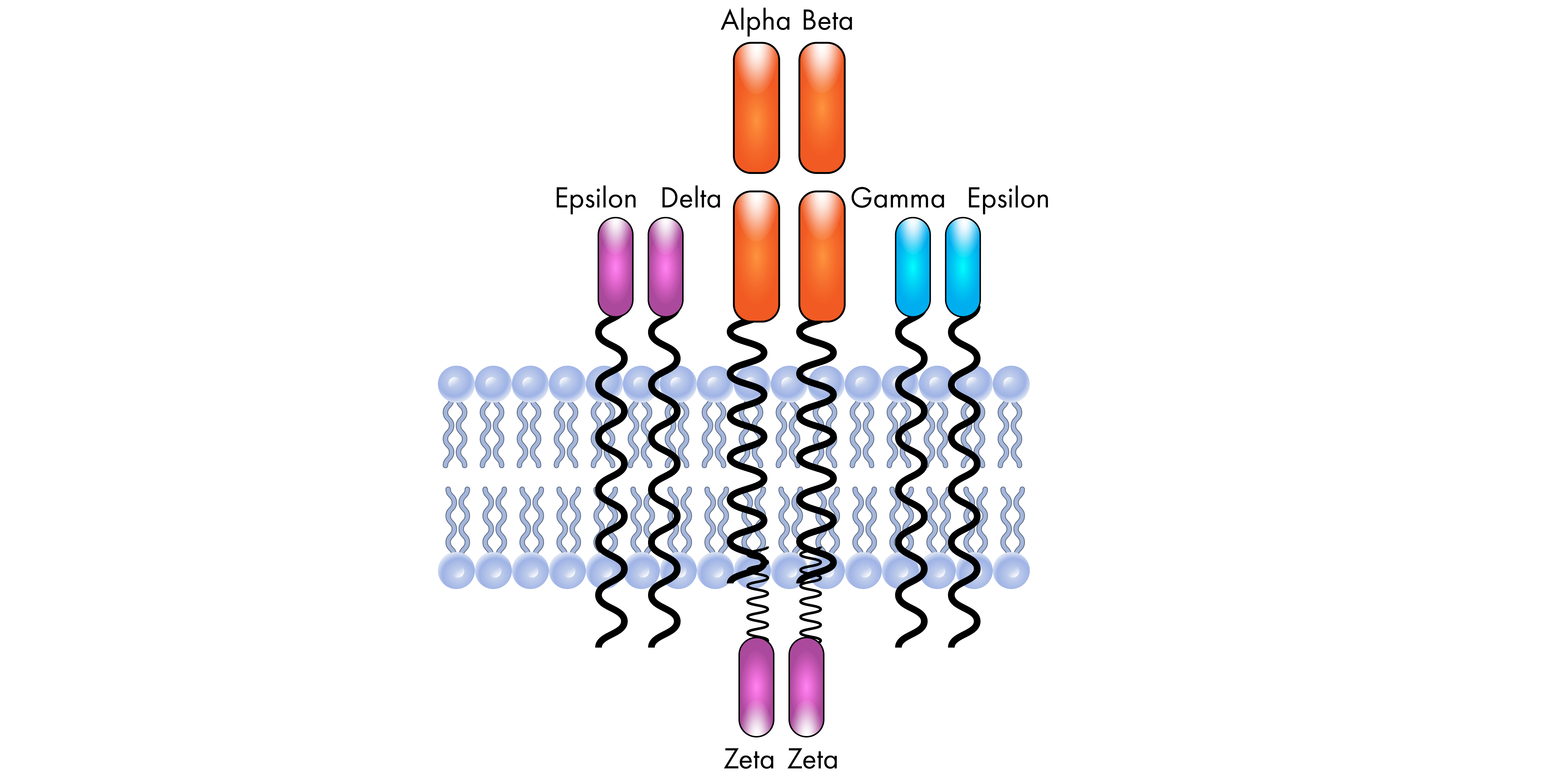
Antibody-based immune checkpoint inhibitors remain a major focus of immuno-oncology drug research and development efforts because of their recent success in providing long-term anti-tumor responses. However, the range of response of different tumor types to these drugs is hugely varied. Small molecule kinase inhibitors that block signaling pathways involved in regulation of tumor immunity at multiple points in the “cancer immunity cycle” may provide alternate, effective therapeutics. One kinase that may be a target for such small molecule inhibitors is Hematopoietic Progenitor Kinase 1 or HPK1; the potential of this kinase as a therapeutic target was reviewed by Sawasdikosol and Burakoff (1). HPK1, also known as MAP4K1, is a member of the MAP kinase protein kinase family that negatively regulates signal transduction in T-cells, B-cells and dendritic cells of the immune system.