It’s been just over 10 years since the world lost a pioneering immunologist and biochemist, Dr. Jürg Tschopp. He died tragically during a hiking trip in the Swiss Alps on March 22, 2011. A host of academic journals, including Science, Nature and Cell, paid tribute to Dr. Tschopp with obituaries that highlighted his many accomplishments in the fields of apoptosis and immunology.
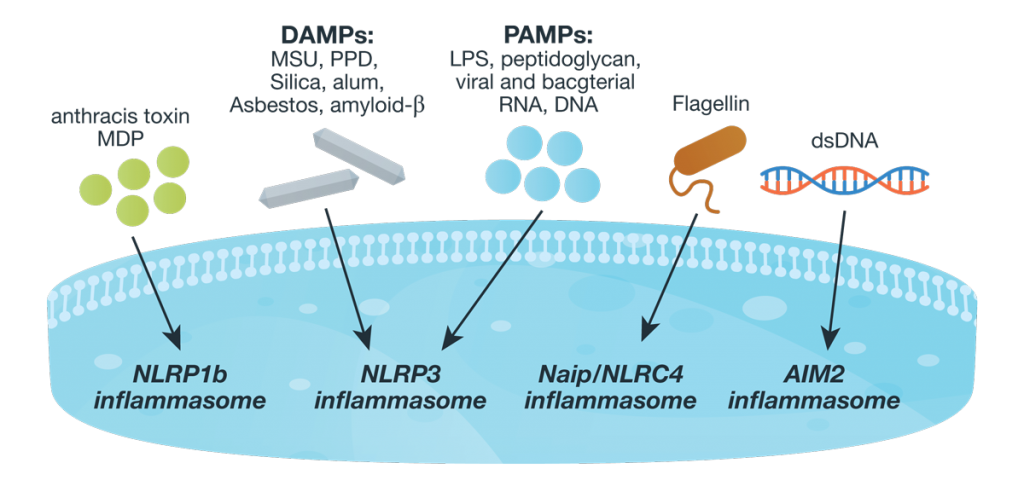
In 2002, a team led by Dr. Tschopp at the University of Lausanne, Switzerland, was studying the role of the proinflammatory cytokine interleukin 1 beta (IL-1β). This cytokine is produced in the cytoplasm as an inactive precursor (pro-IL-1β). It is cleaved by caspase-1 to the active form, but the exact process by which caspase-1 itself is activated was unknown at the time. Several members of the caspase family contain a conserved region known as the caspase recruitment domain or CARD, and it was proposed that this domain was essential to caspase activation.
Based on similarity to another protein containing an N-terminal CARD motif (Apaf-1) that is involved in activation of caspase-9, the researchers examined the roles of a family of proteins known as NALP1, NALP2 and NALP3 (1). In particular, they were interested in NALP1, which is involved in the immune response. Unlike Apaf-1, NALP1 contains a CARD motif at the C terminus, while the N terminus contains a related motif known as a pyrin-like domain (PYD). The research team had previously showed that the PYD region of NALP1 interacted with an adapter protein known as PYCARD or ASC, which also contains an N-terminal PYD and C-terminal CARD.
The results of the team’s in vitro binding, activation and immunodetection studies showed that a multi-unit protein complex is responsible for caspase activation, and they called this complex the “inflammasome” (1). It is composed of caspase-1, caspase-5, PYCARD/ASC and NALP1.
Continue reading “The NLRP3 Inflammasome: Flipping the Switch”
 Anyone who has travelled across time zones knows how unpleasant it is when the regular rhythm of your biological clock is disrupted. Jetlag results when the body’s internal clock, or circadian rhythm is out of sync with external cues for “day and “night”, resulting in insomnia, extreme tiredness, difficulty concentrating and various other unpleasant symptoms.
Anyone who has travelled across time zones knows how unpleasant it is when the regular rhythm of your biological clock is disrupted. Jetlag results when the body’s internal clock, or circadian rhythm is out of sync with external cues for “day and “night”, resulting in insomnia, extreme tiredness, difficulty concentrating and various other unpleasant symptoms. 