It’s been just over 10 years since the world lost a pioneering immunologist and biochemist, Dr. Jürg Tschopp. He died tragically during a hiking trip in the Swiss Alps on March 22, 2011. A host of academic journals, including Science, Nature and Cell, paid tribute to Dr. Tschopp with obituaries that highlighted his many accomplishments in the fields of apoptosis and immunology.
In 2002, a team led by Dr. Tschopp at the University of Lausanne, Switzerland, was studying the role of the proinflammatory cytokine interleukin 1 beta (IL-1β). This cytokine is produced in the cytoplasm as an inactive precursor (pro-IL-1β). It is cleaved by caspase-1 to the active form, but the exact process by which caspase-1 itself is activated was unknown at the time. Several members of the caspase family contain a conserved region known as the caspase recruitment domain or CARD, and it was proposed that this domain was essential to caspase activation.
Based on similarity to another protein containing an N-terminal CARD motif (Apaf-1) that is involved in activation of caspase-9, the researchers examined the roles of a family of proteins known as NALP1, NALP2 and NALP3 (1). In particular, they were interested in NALP1, which is involved in the immune response. Unlike Apaf-1, NALP1 contains a CARD motif at the C terminus, while the N terminus contains a related motif known as a pyrin-like domain (PYD). The research team had previously showed that the PYD region of NALP1 interacted with an adapter protein known as PYCARD or ASC, which also contains an N-terminal PYD and C-terminal CARD.
The results of the team’s in vitro binding, activation and immunodetection studies showed that a multi-unit protein complex is responsible for caspase activation, and they called this complex the “inflammasome” (1). It is composed of caspase-1, caspase-5, PYCARD/ASC and NALP1.
Structure and Function
Today, inflammasomes have become a central concept in the study of the immune system, and dysregulation of inflammasomes is associated with a variety of diseases, from type 2 diabetes to Alzheimer’s disease. The NALP subfamily of proteins studied by Dr. Tschopp are now known to constitute part of the nucleotide-binding oligomerization domain (NOD)-like receptor (NLR) family of inflammasome-associated proteins; currently, at least 23 members are known. Of these, NLRP3 and its corresponding inflammasome have received the greatest attention (reviewed in 2).
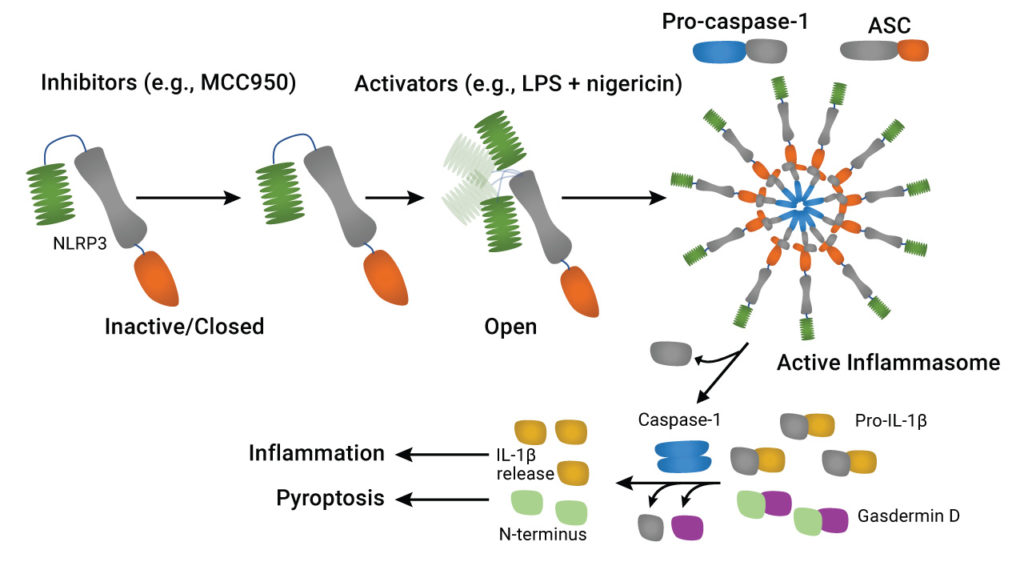
NLRP3 contains three distinct domains. The N-terminal PYD interacts with the corresponding region of ASC. The central NACHT domain (3) is responsible for binding ATP and conversion to ADP, thereby inducing oligomerization of the protein (4). The C-terminal leucine-rich repeat (LRR) domain was originally thought to be necessary for activation of NLRP3 and subsequent inflammasome assembly, but this role for the LRR has been disputed (5).
Activation of the NLRP3 inflammasome is a complex process, although it can be broken down into two characteristic steps (2). The first, “priming” step (Signal 1) is initiated by several pathways involving cytokines or pattern recognition receptors and results in transcriptional activation of NLRP3 and its inflammasome components. The second step (activation or Signal 2) is triggered by a variety of pathogen-associated molecular patterns (PAMPs) and tissue damage-associated molecular patterns (DAMPs), from RNA viruses to reactive oxygen species (2). Further complexity is introduced by transcriptional regulation and post-translational modifications of inflammasome components; however, the end result is assembly of the NLRP3 inflammasome. This protein complex activates caspase-1, followed by conversion of pro-IL-1β and pro-IL-18 to the active cytokines. Another important consequence of caspase-1 activation is a caspase-dependent form of apoptosis (programmed cell death) known as pyroptosis, mediated by cleavage of the protein gasdermin D (6). Pyroptosis can be distinguished from apoptosis by multiplexing cell-based assays that measure caspase-1 activity together with cell death in real time (7).
Therapeutic Targets
Since NLRP3 inflammasome activation is implicated in a wide range of infectious and inflammatory diseases (reviewed in 8), considerable research been devoted to developing therapeutic agents that target specific steps in the NLRP3 pathway. These include (8):
- inhibitors of NLRP3 activation, such as MCC950, MNS and OLT1177 that target the NACHT domain;
- ASC inhibitors, such as caffeic acid phenethyl ester;
- caspase-1 inhibitors, such as VX-740 and VX-765
- IL-1β inhibitors, such as the IL-1β receptor antagonist anakinra, and monoclonal antibody therapeutics canakinumab and gevokizumab;
- various indirect inhibitors that target points upstream and downstream of the NLRP3 activation pathway.
Although none of these therapies have currently made it into the clinic, the ongoing process of drug discovery and development holds promise for future treatment of a broad spectrum of inflammatory diseases. Further research into understanding the complex pathways involved in NLRP3 inflammasome activation will help to refine these therapies and develop highly specific therapeutics with minimal off-target effects. These efforts will bring to fruition the vision of Dr. Tschopp when he coined the word “inflammasome” 20 years ago.
Learn more about inflammasome activation with our Inflammasome Activation Drug Discovery page.
References
- Martinon, F. et al. (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of pro IL-1b. Mol. Cell 10, 417–426.
- Paik, S. et al. (2021) An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell. Mol. Immunol. 18, 1141–1160.
- Koonin, E.V. and Aravind, L. (2000) The NACHT family – a new group of predicted NTPases implicated in apoptosis and MHC transcription activation. Trends Biochem. Sci. 25, 223–224.
- Broz, P. et al. (2016) Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 16, 407–420.
- Hafner-Bratkovič, I. et al. (2018) NLRP3 lacking the leucine-rich repeat domain can be fully activated via the canonical inflammasome pathway. Nat. Comm. 9, 5182.
- Bai, B. et al. (2020) NLRP3 inflammasome in endothelial dysfunction. Cell Death Dis. 11, 776.
- O’Brien, M. et al. (2017) A bioluminescent caspase-1 activity assay rapidly monitors inflammasome activation in cells. J. Immunol. Meth. 447, 1–13.
- Seok, J.K. et al. (2021) Therapeutic regulation of the NLRP3 inflammasome in chronic inflammatory diseases. Arch. Pharm. Res. 44, 16–35.
Related Posts
Latest posts by Ken Doyle (see all)
- Will Artificial Intelligence (AI) Transform the Future of Life Science Research? - February 1, 2024
- RAF Inhibitors: Quantifying Drug-Target Occupancy at Active RAS-RAF Complexes in Live Cells - September 5, 2023
- Synthetic Biology: Minimal Cell, Maximal Opportunity - July 25, 2023
