Insects are a keystone species in the animal kingdom, often providing invaluable benefits to terrestrial ecosystems and useful services to mankind. While many of them are seen as pests (think mosquitos), others are important for pollination, waste management, and even scientific research.
Insect biotechnology, or the use of insect-derived molecules and cells to develop products, is applied in a diverse set of scientific fields including agricultural, industrial, and medical biotechnology. Insect cells have been central to many scientific advances, being utilized in recombinant protein, baculovirus, and vaccine and viral pesticide production, among other applications (5).
Therefore, as the use of insect cells becomes more widespread, understanding how they are produced, their research applications, and the scientific products that can be used with them is crucial to fostering further scientific advancements.
Primary Cell Cultures and Cell Lines

In general, experimentation with individual cells, rather than full animal models, is advantageous due to improved reproducibility, decreased space requirements, less ethical concerns, and a reduction in expense. This makes primary cell cultures and cell lines essential contributors to basic scientific research.
Continue reading “Insects and Science: Optimizing Work with Sf9 Insect Cells”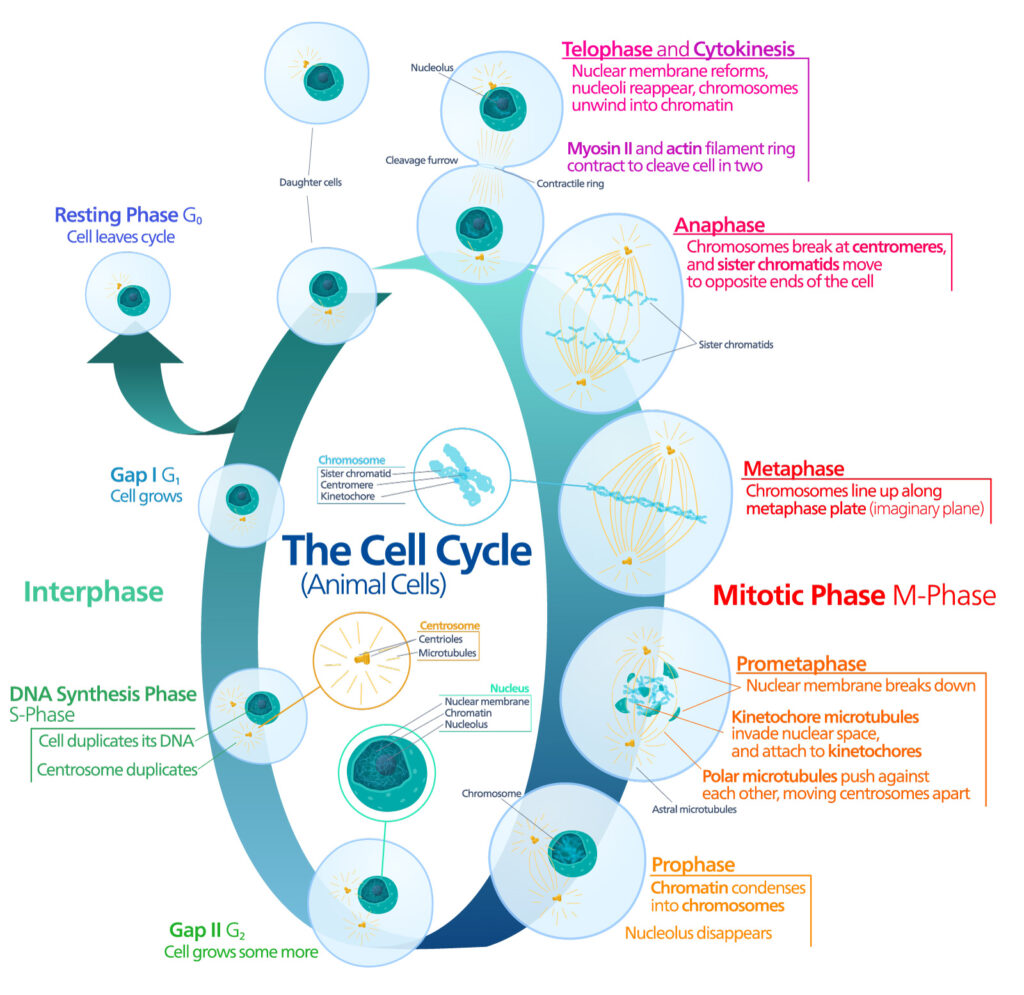
 What if you could uncover a small but significant cellular response as your population of cells move toward apoptosis or necrosis? What if you could view the full picture of cellular changes rather than a single snapshot at one point? You can! There are real-time assays that can look at the kinetics of changes in cell viability, apoptosis, necrosis and cytotoxicity—all in a plate-based format. Seeking more information? Multiplex a real-time assay with endpoint analysis. From molecular profiling to complementary assays (e.g., an endpoint cell viability assay paired with a real-time apoptosis assay), you can discover more information hidden in the same cells during the same experiment.
What if you could uncover a small but significant cellular response as your population of cells move toward apoptosis or necrosis? What if you could view the full picture of cellular changes rather than a single snapshot at one point? You can! There are real-time assays that can look at the kinetics of changes in cell viability, apoptosis, necrosis and cytotoxicity—all in a plate-based format. Seeking more information? Multiplex a real-time assay with endpoint analysis. From molecular profiling to complementary assays (e.g., an endpoint cell viability assay paired with a real-time apoptosis assay), you can discover more information hidden in the same cells during the same experiment.

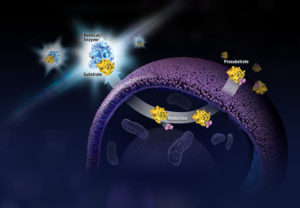
 Life is complicated. So is death. And when the cells in your multiwell plate die after compound treatment, it’s not enough to know that they died. You need to know how they died: apoptosis or necrosis? Or, have you really just reduced viability, rather than induced death? Is the cytotoxicity you see dose-dependent? If you look earlier during drug treatment of your cells, do you see markers of apoptosis? If you wait longer, do you observe necrosis? If you reduce the dosage of your test compound, is it still cytotoxic?
Life is complicated. So is death. And when the cells in your multiwell plate die after compound treatment, it’s not enough to know that they died. You need to know how they died: apoptosis or necrosis? Or, have you really just reduced viability, rather than induced death? Is the cytotoxicity you see dose-dependent? If you look earlier during drug treatment of your cells, do you see markers of apoptosis? If you wait longer, do you observe necrosis? If you reduce the dosage of your test compound, is it still cytotoxic? 