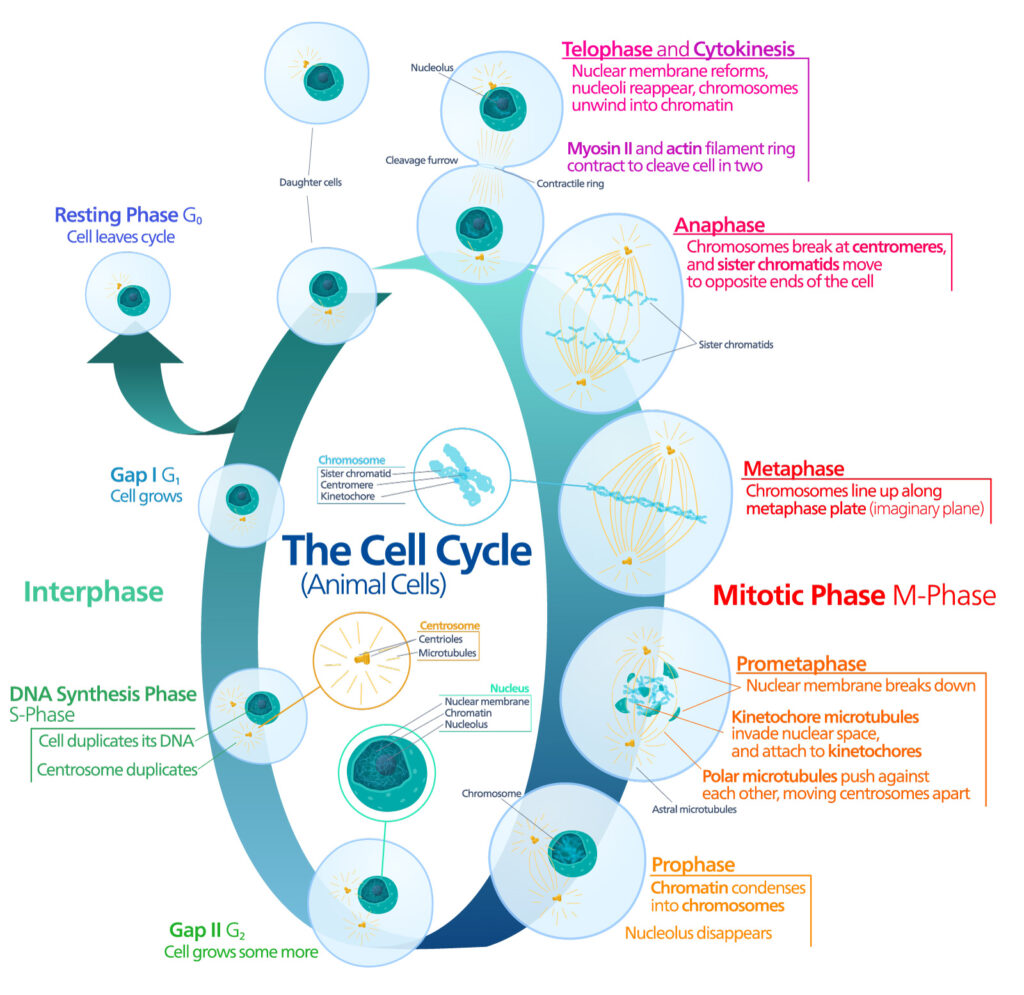
Introduction
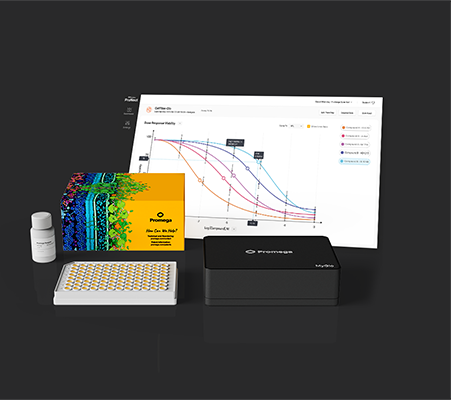
When it comes to laboratory tools, few things resonate more than the experiences of researchers who rely on them daily. At the University of Cincinnati the MyGlo Reagent Reader has quickly become an indispensable lab companion, due to its compact design, affordability, and intuitive interface with tailored apps for Promega assays. But what truly sets the MyGlo Reagent Reader apart is how it empowers scientists to focus on their research.
Take Ipsita Kundu, a third-year PhD student at the University of Cincinnati working in Dr. Tim Phoenix’s lab. The Phoenix lab, dedicated to studying innovative brain tumor therapies, faced challenges with their outdated luminescence reader. They needed an affordable, reliable solution to streamline Ipsita’s experiments without compromising accuracy or efficiency.
The MyGlo Reagent reader is Nominated for a 2025 Select Science, Scientists’ Choice Award in the category of Life Sciences Product of the Year. Do you agree that it is a game changer? Vote today!
The MyGlo Reagent Reader was the answer. This blog highlights how this integrated solution is redefining laboratory workflows, enabling researchers to maximize productivity and maintain focus on groundbreaking discoveries. Let’s delve into Ipsita’s story and explore how MyGlo transformed her research.
Continue reading “Glo-ing Above and Beyond: Simplifying Science with MyGlo Reagent Reader”


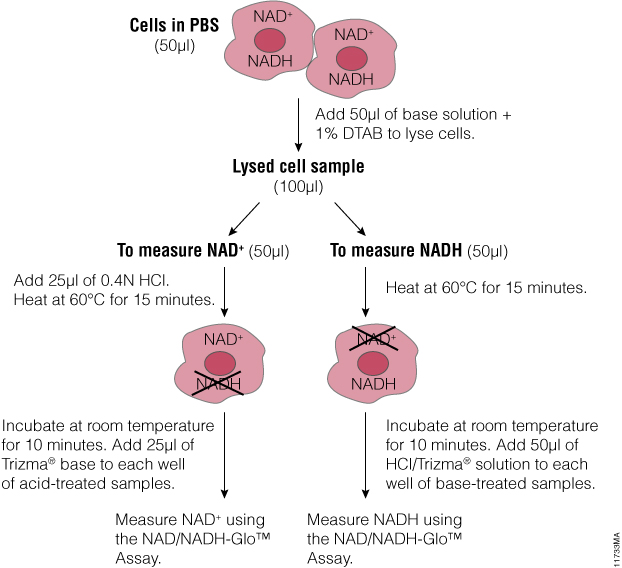
 When someone is admitted to a hospital for an illness, the hope is that medical care and treatment will help them them feel better. However, nosocomial infections—infections acquired in a health-care setting—are becoming more prevalent and are associated with an increased mortality rate worldwide. This is largely due to the misuse of antibiotics, allowing some bacteria to become resistant. Furthermore, when an antibiotic wipes out the “good” bacteria that comprise the human microbiome, it leaves a patient vulnerable to opportunistic infections that take advantage of disruptions to the gut microbiota.
When someone is admitted to a hospital for an illness, the hope is that medical care and treatment will help them them feel better. However, nosocomial infections—infections acquired in a health-care setting—are becoming more prevalent and are associated with an increased mortality rate worldwide. This is largely due to the misuse of antibiotics, allowing some bacteria to become resistant. Furthermore, when an antibiotic wipes out the “good” bacteria that comprise the human microbiome, it leaves a patient vulnerable to opportunistic infections that take advantage of disruptions to the gut microbiota.