Mix a love of eating with a desire to live a long, healthy life what do you get? Probably the average 21st-century person looking for a way to continue enjoying food despite insufficient exercise and/or an age-related decline in caloric needs.
Enter intermittent fasting, a topic that has found its way into most news sources, from National Institutes of Health (NIH) and Proceedings of the National Academy of Sciences publications to WebMD and even the popular press. For instance, National Public Radio’s “The Salt” writers have tried and written about their experiences with dietary restriction.
While fasting has enjoyed fad-like popularity over the past several years, it is not new. Fasting, whether purposely not eating or eating a restricted diet, has been practiced for 1,000s of years. What is new is research studies from which we are learning the physiologic effects of fasting and other forms of decreased nutrient intake.
You may have heard the claims that fasting makes people smarter, more focused, and thinner. Researchers today are using cell and animal models, and even human subjects, to measure biochemical responses at the cellular level to restricted nutrient intake and meal timing, in part to prove/disprove such claims (1,2).
Continue reading “In Healthy Eating Less is More: The Science Behind Intermittent Fasting” Like this:
Like Loading...

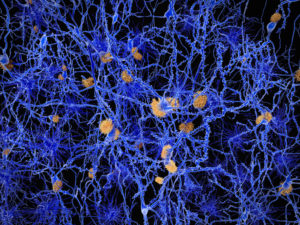
 Back in 2015 the Ice Bucket Challenge brought Amyotrophic Lateral Sclerosis (ALS) to public attention, initiating worldwide pleas for more funding of research toward a cure for this fatal disease, which is characterized by progressive degeneration of motor neurons. In spite of many efforts over the last few decades, the precise cause of ALS is still unknown.
Back in 2015 the Ice Bucket Challenge brought Amyotrophic Lateral Sclerosis (ALS) to public attention, initiating worldwide pleas for more funding of research toward a cure for this fatal disease, which is characterized by progressive degeneration of motor neurons. In spite of many efforts over the last few decades, the precise cause of ALS is still unknown.
