A Bioluminescent Alternative
Fluorescence resonance energy transfer (FRET) probes or sensors are commonly used to measure cellular events. The probes typically have a matched pair of fluorescent proteins joined by a ligand-binding or responsive protein domain. Changes in the responsive domain are reflected in conformational changes that either bring the two fluorescent proteins together or drive them apart. The sensors are measured by hitting the most blue-shifted fluorescent protein with its excitation wavelength (donor). The resulting emission is transferred to the most red-shifted fluorescent protein in the pair, and the result is ultimately emission from the red-shifted protein (acceptor).
As pointed out by Aper, S.J.A. et al. below, FRET sensors face challenges of photobleaching, autofluorescence, and, in the case of exciting cyan-excitable donors, phototoxicity. Another challenge to using FRET sensors comes when employing optogenetic regulators to initiate the event you wish to monitor. Optogenetic regulators respond to specific wavelengths and initiate signaling. The challenge comes when the FRET donor excitation overlaps with the optogenetic initiation wavelengths. Researchers have sought to alleviate many of these challenges by exchanging the fluorescent donor for a bioluminescent donor, making bioluminescence resonance energy transfer (BRET) probes. In the three papers described below, the authors chose NanoLuc® Luciferase as the BRET donor due to its extremely bright signal.
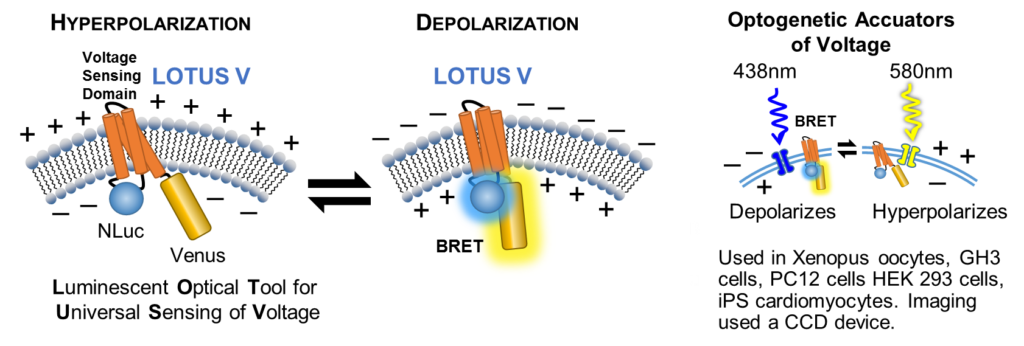
Live-Cell Voltage Sensor (LOTUS V)
Ingaki and colleagues were interested in using photoactivatable, optogenetic accuators to depolarize or hyperpolarize membranes. Traditional fluorescent FRET reporters could not be used as the excitation wavelengths needed would also activate the optogenetic accuators. The researchers took a fluorescent FRET reporter and adapted it into a BRET reporter by using NanoLuc® Luciferase to excite the Venus fluorescent protein in conjunction with a membrane-inserted voltage sensing domain (LOTUS V; Luminescent Optical Tool for Universal Sensing of Voltage). Depolarized membranes allow the BRET reaction and hyperpolarized membranes separate the two and no BRET occurs. The authors demonstrate how LOTUS V works in many model systems. This construct is available through Addgene (Cat. # 87127).
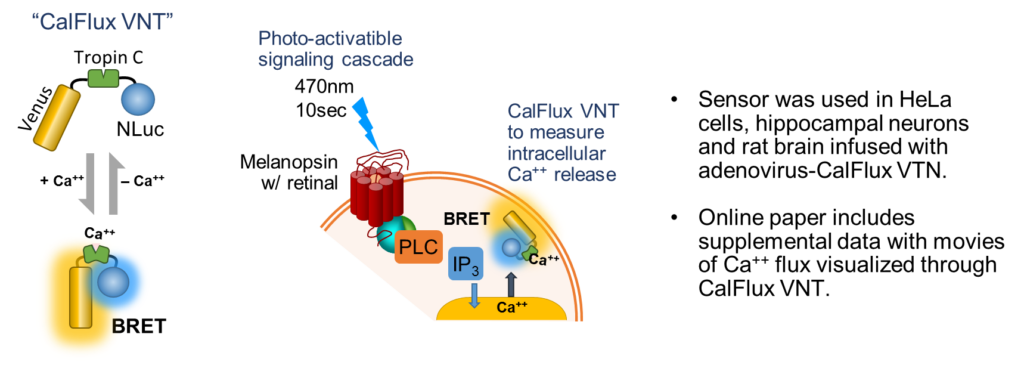
Intracellular Calcium Sensor (CalFlux VNT)
Yang, J. et al. wished to trigger intracellular Ca++ release with a optogenetic receptor, melanopsin, requiring brief exposure to 470nm blue light. A probe was needed that had some very specific characteristics as outlined by the authors: did not require photoexcitation, was ion-specific, sensitive, expressible and gave ratiometric data. The result was a molecule called CalFlux VNT. CalFlux VNT is composed of Venus fluorescent protein, Troponin C Ca++ binding domain, and NanoLuc® Luciferase. The CalFlux VNT responded to changes in intracellular calcium and was useful for direct imaging of the events in cells and brain explants. This lab (Carl H. Johnson, Vanderbilt University) invented the BRET technique. This construct is available through Addgene (Cat. #83926).
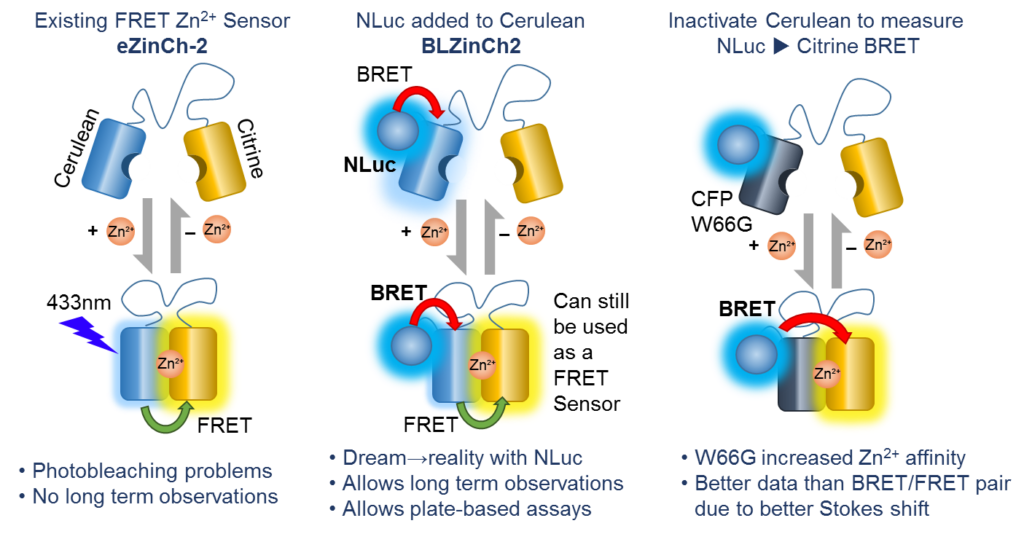
Intracellular BRET/FRET Zinc Sensor (BL-ZinCh)
Aper and colleagues wanted to adapt an existing FRET sensor to use a bioluminescent excitation source to avoid photobleaching, phototoxicity, autofluorescence, etc. A FRET-based sensor (Cerulean→Citrine; eZinCh) for measuring intracellular Zn2+ was appended with NanoLuc® Luciferase to make a sensor that could use either BRET (NLuc→Cerulean→Citrine or NLuc→Citrine; BL-ZinCh) or FRET for detection of intracellular Zn2+. The sensor was used in HeLa cells to monitor changes in Zn2+ levels in both plate-based and imaging-based analysis. This construct is available through Addgene (Cat. #85082).
Pairing NanoLuc® Luciferase with fluorescent proteins has allowed researchers to make innovative tools not possible with other luciferases. These papers also provide a blueprint for others to apply NanoLuc® Luciferase and fluorescent protein to make more and more tools to aid their research. All three groups deposited sensor clones in Addgene (www.addgene.org) , a non-profit plasmid repository. Researchers around the world can obtain these tools for a small fee and employ them in their lab immediately.
Literature Cited
- Aper, S.J.A. et al. (2016) Dual-readout BRET/FRET sensors for measuring intracellular zinc. ACS Chem. Biol. 11, 2854-64. PubMed ID: 27547982
- Inagaki, S., et al. (2017) Genetically encoded bioluminescent voltage indicator for multi-purpose use in wide range of bioimaging. Sci. Reports 7, 42398. PubMed ID: 28205521
- Yang, J., et al. (2016) Coupling optogenetic stimulation with NanoLuc-based luminescence (BRET) Ca++ sensing. Nature Comm. 7, 13268. PubMed ID: 27786307
Questions about NanoLuc® Luciferase or BRET? Contact a Promega Technical Services Scientist, techserv@promega.com.
