Oncologists, do you know your colorectal cancer patients’ MSI status?
High-frequency microsatellite instability (MSI-H) in tumors is a form of genomic instability where mismatch repair (MMR) proteins fail to properly correct errors in microsatellite regions of the genome. When a patient’s tumor tissue is determined to have MSI-H markers, it’s strongly recommended that they be further tested for Lynch syndrome, a hereditary condition that puts them and their family at a higher risk of developing colorectal and other cancers (1).
Though as many as 1 in 279 people might be carriers for the mutations associated with Lynch syndrome (2), 95% of them don’t know it (3). Furthermore, people with Lynch syndrome have an approximately 80% increased lifetime risk of developing colorectal cancer, compared to a risk of only ~4% for the general population (4, 5).
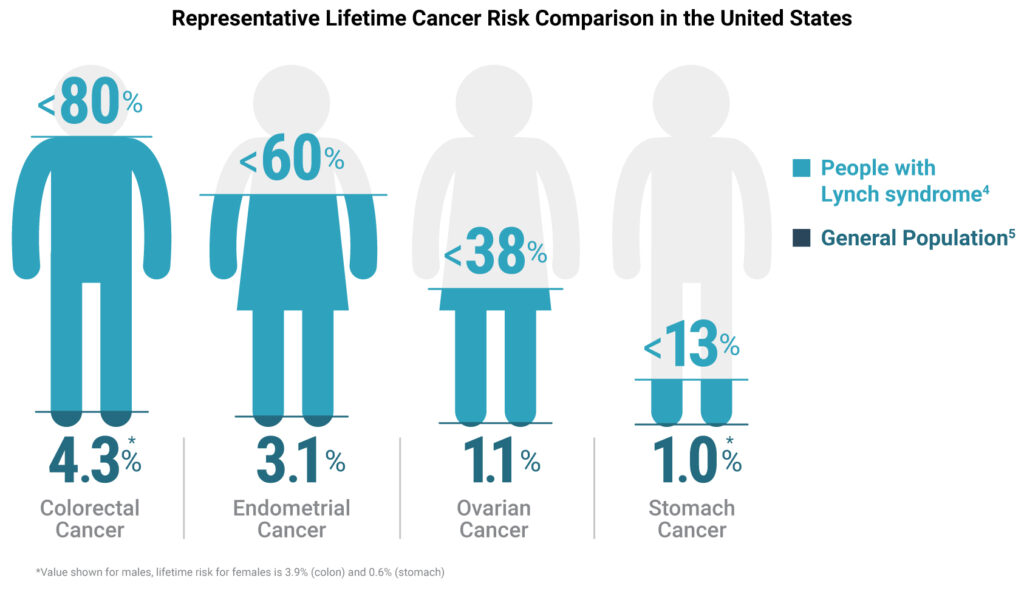
On Lynch Syndrome Awareness Day, here are three key reasons why you should test all your colorectal cancer patients’ MSI status.
1. MSI Status Can Inform How You Approach Patient Care
Defective expression of MMR proteins can produce immunogenic proteins that are susceptible to immunotherapies. An MSI-H status for a patient’s tumor tissue can predict if their cancer will respond to immunotherapies like immune checkpoint blockade inhibitors. Knowing your patients’ MSI status can help you design a well-informed screening and treatment plan that meets their and their families’ specific needs.
Read more about MSI status as a predictor of therapeutic responses.
2. For Your Patients, Knowledge is Power
When a person develops recurring primary cancers or when cancer emerges in each generation of a family tree, it can feel like a curse. But, if your patients know that they have Lynch syndrome, they are better able to understand their own health and make informed choices about their care. They can also encourage family members to be tested.

Knowing they live with a hereditary condition that puts them and family members at greater risk of developing cancer can create opportunities for your patients to make different lifestyle choices, seek out therapy or speak candidly about the future with their loved ones.
Read how genetic testing empowered one patient with a Lynch syndrome diagnosis.
“Part of why I went through everything I’ve been through is because I didn’t know about Lynch. I want to get the word out. Your knowledge is power, and it’s going to keep you healthy and safe.”
Carrie Ketcham, Lynch syndrome patient
3. MSI Detection Methods are Relatively Inexpensive and Highly Accurate
The two most common methods for screening patients for MSI or deficient MMR (dMMR) are PCR-based tests and immunohistochemistry (IHC)-based tests. PCR-based methods identify the DNA-level mutations that arise from deficiencies in the DNA repair process and cost about $45/tissue sample. IHC tests for the absence or presence of MMR proteins and costs about $50–70/slide.
When you order parallel PCR- and IHC-based tests for your cancer patients, you’ll be able to correctly characterize their tumors with over 99% accuracy (6).
Read more about methods for detecting MSI.
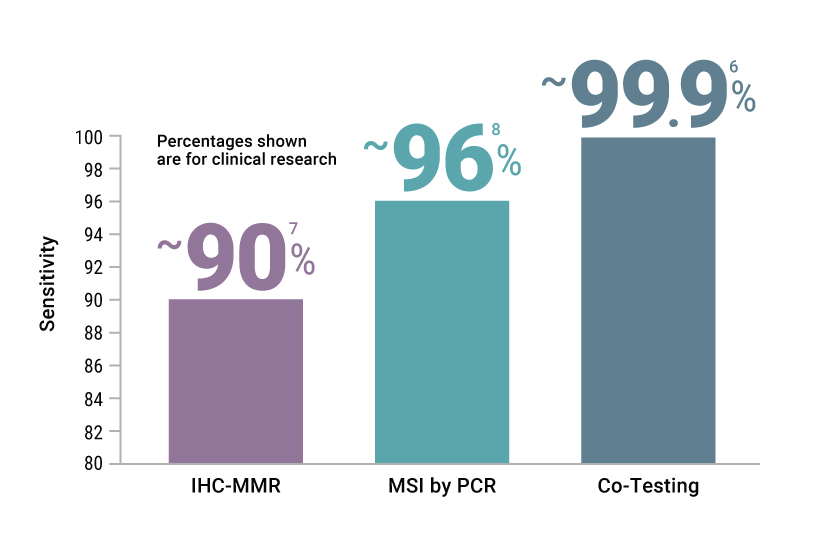
PCR-based Tests Provide Fast and Accurate Detection of MSI Status
At Promega, we’re committed to supporting the medical community in their ongoing work to provide patients with the best care possible.
While IHC tests can detect only the absence or presence of MMR proteins, PCR-based tests like our OncoMate® MSI Dx Analysis System directly measure the genomic mutations that arise from functional loss of MMR protein function. The OncoMate MSI Dx Analysis System is indicated in patients diagnosed with colorectal cancer (CRC) to detect microsatellite instability (MSI) as an aid in the identification of probably Lynch syndrome to help identify patients that would benefit from additional genetic testing do diagnose Lynch syndrome. The test can reliably identify MSI status of your colorectal cancer patients’ tumor samples in as little as ~10 hours.
The clinical performance of this device to guide treatment decisions for MSI-H patients has not been established.
Learn more about how the FDA-cleared OncoMate® MSI System can help you make more informed decisions with your patients.
References
- Mao, R. et al (2021) Genetic Testing for Inherited Colorectal Cancer and Polyposis, 2021 Revision: A Technical Standard of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 23(10), 1807–1817.
- Win, A.K. et al. (2016) Prevalence and Penetrance of Major Genes and Polygenes for Colorectal Cancer. Cancer Epidemiol. Biomarkers Prev. 26(3), 404–412.
- Cancer.gov. Cancer MoonshotSM Blue Ribbon Panel Report. Accessed March 3, 2023.
- Cancer.net. Lynch Syndrome. Accessed March 3, 2023.
- Cancer.org. Lifetime Risk of Developing or Dying from Cancer. Accessed March 3, 2023.
- Funkhouser, W.K. Jr. et al. (2012) Relevance, Pathogenesis, and Testing Algorithm for Mismatch Repair–Defective Colorectal Carcinomas: A Report of the Association for Molecular Pathology. J. Mol. Diagnostics 14(2), 91–103.
- Dudley, J.C. et al. (2016) Microsatellite Instability as a Biomarker for PD-1 Blockade. Clin. Cancer Res. 22, 813–820.
- Based on an internal analysis of publications comparing MSI-PCR v. IHC-dMMR in colorectal cancer from 2004–2018. Literature bundle available from Promega Medical Affairs upon request.
Related Posts
Latest posts by Jordan Nutting (see all)
- The Central Dogma of Promega: The Story and Science Behind Our Kit Packaging Design - May 7, 2024
- Silencing the Immunogenicity of AAV Vectors - April 4, 2024
- Discovering Cyclic Peptides with a “One-Pot” Synthesis and Screening Method - February 29, 2024
