We share this planet with approximately 8.7 million species of plants and animals. Within such a diverse environment, it’s only natural that many complex relationships have developed among different species. Some relationships are mutually beneficial, some are parasitic—and some are lethal.

Natural toxins and venoms are biologically active compounds produced by normal metabolic processes in an organism but are harmful to other organisms. Typically, toxins are encountered passively or ingested by the affected organisms, and have a specific mode of action and binding site within a cell. In contrast, venoms are introduced directly into the victim through a specialized delivery mechanism, and they may consist of a mixture of compounds that affect a range of cell types and tissues (1). Both types of poisons are produced for predation, defense, or to offer a competitive advantage (1).
Continue reading “Genome-Wide CRISPR Screening: Putting Death on Hold”



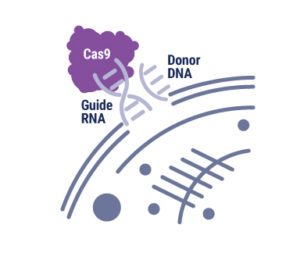
 GPCRs
GPCRs