Cancer vaccines have been in progress for some time now. But a vaccine that is highly effective against cancer is not currently available.
However, an interesting report from Stanford University School of Medicine researchers, Dr. Irving Weissman, et al. shows some promise in a development of an altered means of stimulating the immune system, that could result in a stronger immune response and ultimately a better cancer vaccine. The paper by Weissman et al. was published electronically ahead of print in PNAS USA, May 20, 2013: “Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response.”
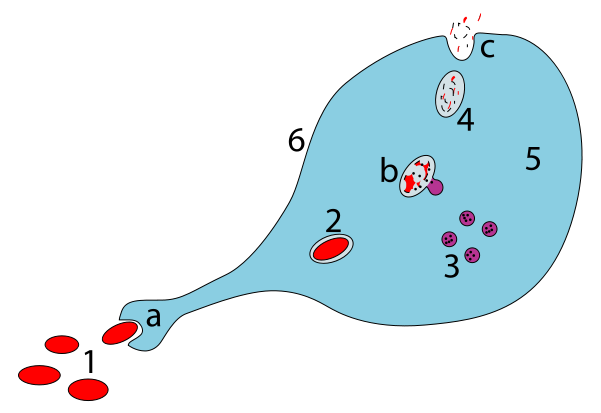
The immune system has two main branches, two lines of attack for recognition and elimination of invaders. When foreign bacterial or viral (or cancer) cells appear in the body they come into contact with the innate immune system, which is composed of macrophages and dendritic cells. These cells, as the image shows, can engulf, digest and eventually repurpose pieces of invading organisms. Macrophages and dendrites, after digesting these invaders, present pieces of the invaders on their cell surface or pass them to the external environment.
Once the pieces appear on the surface of macrophages/dendritic cells, they come into contact with T cells, which represent the adaptive immune response. T cells are stimulated by the presence of these foreign cell pieces or antigens and replicate in massive numbers. There are two main types of T cells, CD4+ helper T cells, and CD8+ T cells, killer T cells. As you might imagine, its the killer T cells that bring the best vaccine response, attacking and killing foreign organisms. T cells also release cytokines and other chemicals that act to ramp up the immune response.
However, the end result of phagocytosis, its success, may depend on the type of cell that engulfs the pathogen or offending cells.
As shown by S. Burgdorf et al. (1), mannose receptor-mediated endocytosis on dendritic cells was associated with MHC I antigen presentation, while scavenger receptor-mediated endocytosis has been associated with MHC II presentation.
In addition, the outcome of antigen presentation has been shown to depend on the environment in which it occurs. L.C. Bonifaz et al. (2) showed that the dendritic cell receptor for endocytosis, when targeted under noninflammatory conditions (no T cells, no cytokine release) caused induction of tolerance, whereas in the presence of cytokines and T cells, an immune-mediated response resulted.
Dendritic cells have been the focus of many recent attempts at triggering T cell proliferation, in particular, proliferation of CD8+ T cells, known as killer T cells. But use of dendritic cells as antigen presenters has resulted in only modest T cell response to cancer cells.
CD47 is a cell surface molecule that is highly expressed on the surface of cancer cells. This molecule acts as a “don’t eat me” signal to macrophages. Weissman et al. was able to block this signal using an anti-CD47 antibody. They demonstrated the therapeutic ability of anti-CD47 monoclonal antibodies against xenograft human cancer, in mice. Cancer types tested included lymphoma, leukemia and multiple myeloma, but also against solid tumors including prostate, breast, colon and bladder cancers.
In addition, the Weissman group saw a tremendous increase in activation of CD8+ T killer cells, when using their anti-CD47 monoclonal antibody with macrophages.
Results of this study are so encouraging that the Weissman group expects to start human clinical trials of anti-CD47 antibody therapy in 2014.
References
- Burgdorf, S. et al. (2007) Distinct pathways of antigen uptake adn intracellular routing in CD4 and CD8 T cell activation. Science 316, 612-6.
- Bonifaz, L.C. et al. (2004) In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J. Exp. Med. 199, 815-24.
Kari Kenefick
Latest posts by Kari Kenefick (see all)
- Fluorescent Ligands in Biological Research: Where We’ve Been, Where We’re Headed - June 27, 2024
- Cell-Based Target Engagement and Functional Assays for NLRP3 Inhibitor Profiling Help Identify Successes and Failures - January 25, 2024
- Illuminating the Brain with a New Bioluminescence Imaging Substrate - April 13, 2023
