A recent article published in Cancers demonstrates a new method for targeting glial cells using a lentiviral packaging system that incorporated Zika virus envelope proteins. By using the reporter gene firefly luciferase, researchers demonstrated that a pseudotyped virus could infect cultured glioblastoma cells.
Introduction

Viruses enjoy a fearsome reputation. SARS-CoV-2 is only the latest infectious agent that has garnered attention by becoming a worldwide pandemic. Even the viral name suggests that SARS-CoV-2 was not the first of its type [SARS-CoV is the virus behind the severe acute respiratory syndrome (SARS) that spread worldwide in the early 2000s]. There are many different families of viruses (e.g., coronavirus for SARS-CoV-2 or lentiviruses for HIV-1) and each show a preference to the cell types they want to infect. By investigating the life cycle of viruses to better understand their mechanisms, researchers can discover new opportunities that may be exploited.
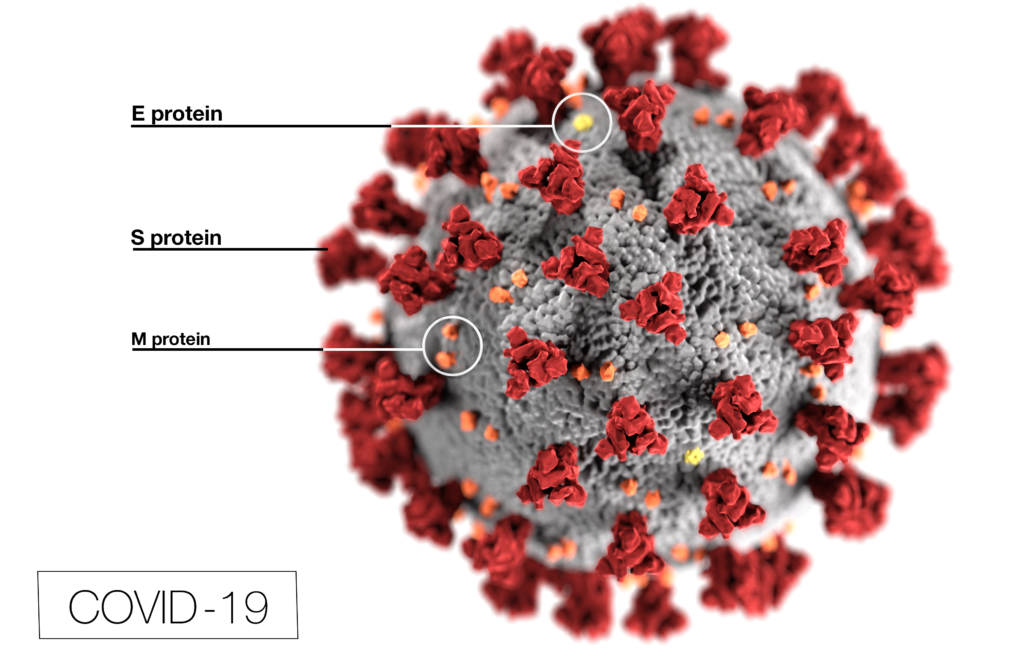
In 2015 and 2016, the virus that concerned health authorities was Zika virus (ZIKV). While this virus generally caused mild disease, the babies of women who were infected during pregnancy were at increased risk for microcephaly and other brain defects. These defects were traced back to Zika virus infecting nerve tissue, specifically, glial cells. This discovery provided an opportunity to explore how Zika virus might affect the brain tumor, glioblastoma multiforme (GMB), especially the glioblastoma stem cells (GSCs) that resist conventional treatment and contribute to the poor prognosis for GMB. Studies suggested that Zika virus infection prolonged survival in animal glioma models and selectively killed GSC with minimal effects on normal cells. In fact, the molecules used by ZIKV to enter cells were predominantly found on tumors, not normal cells. Knowing that the ZIKV envelope proteins prM and E provide the target specificity for glial cells, Kretchmer et al. wanted to explore if ZIKV envelope proteins substituted in lentivirus packaging systems would be able to enter glioblastoma cells.
Continue reading “Targeting Glioblastoma Cells by Packaging a Lentiviral Vector Inside a Zika Virus Coat”