Today’s blog is written by Chuck York, VP of Manufacturing Operations at Promega.
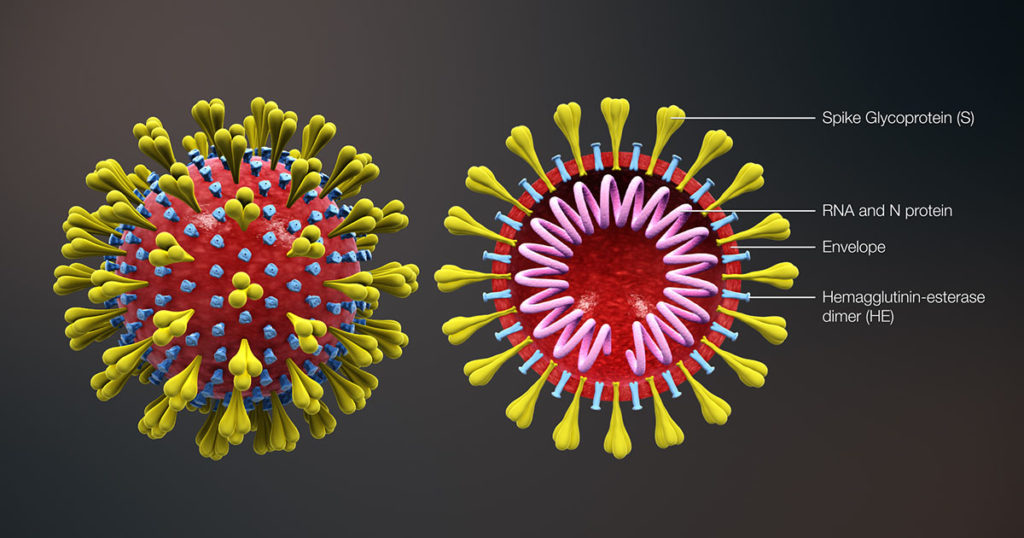
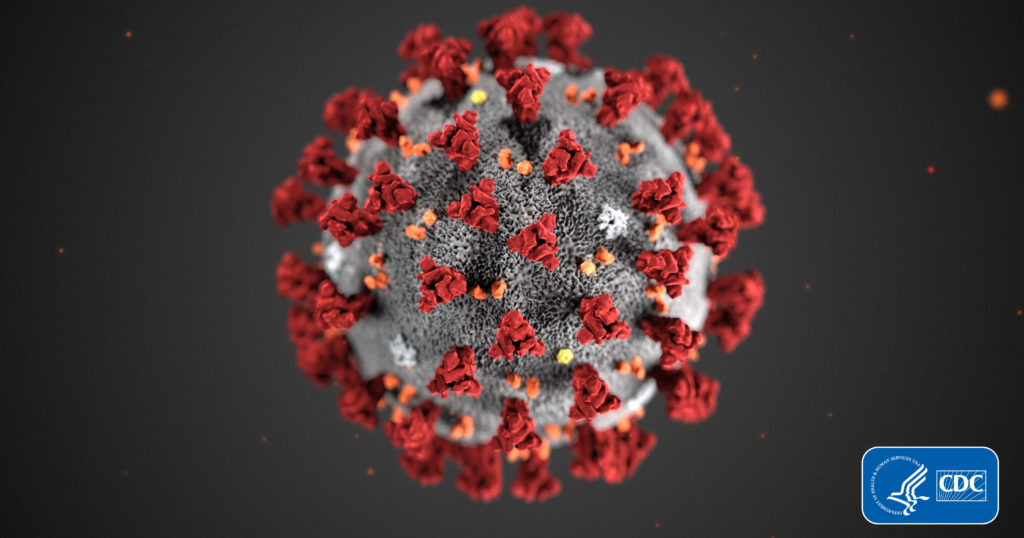
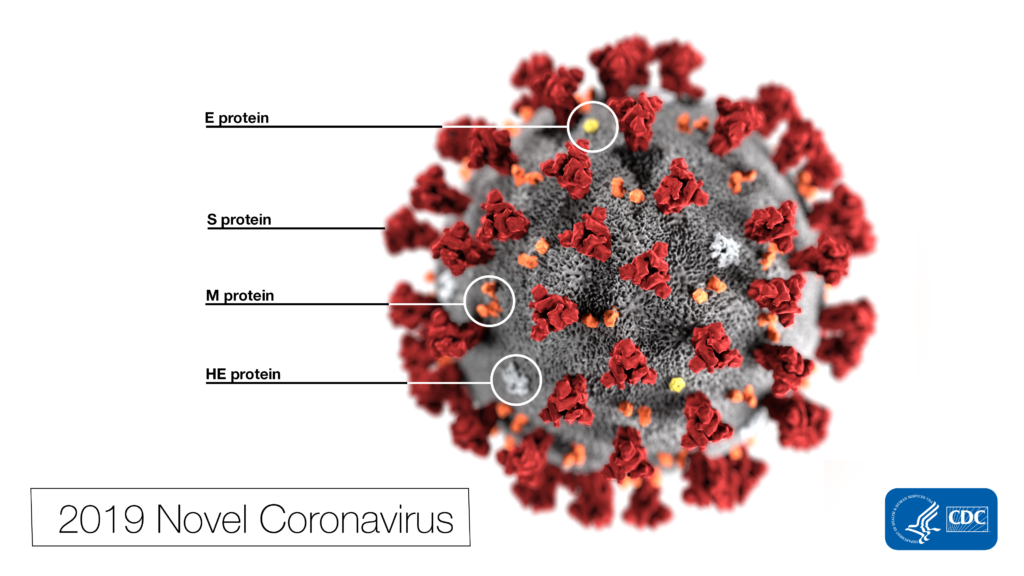
Coronavirus SARS-2-CoV continues to fuel unprecedented demand for COVID-19 related products. Once a term relegated to virology research labs, “coronavirus” is now a household term and a global crisis that has upended lives, disrupted entire economies and shaken our sense of normalcy.
Clinicians, researchers, government officials and the general public are understandably concerned about the availability of reagents for coronavirus testing. At Promega, we are hearing the needs and concerns of our scientific colleagues and partners, and we are doing all that we can to help alleviate them.
At Promega, we are hearing the needs and concerns of our scientific colleagues and partners, and we are doing all that we can to help alleviate them.
As a global company with thousands of products, we have been meeting customer demand in response to market dynamics for decades. Our long-term approach has served customers well. Our efforts to provide support for the COVID-19 response began in early January, with our work with our colleagues and customers in China. We are applying what we’ve learned to propel us forward in the most efficient way now.
We continue to increase production of all COVID-19 related reagents and instruments due to an unprecedented increase in global demand. Production lines that were running one shift 5 days a week are now operating 3 shifts seven days a week, and we continue to take measures to increase our manufacturing capacity.
Continue reading “Meeting Customer Needs in Response to Market Dynamics: Responding to the Coronavirus Pandemic”