Here at Promega, we have been helping your experiments “Glo” for 30 years by utilizing the sensitivity and wide dynamic range of bioluminescence detection methods. However, we’ve found that many scientists are still more familiar with older techniques like colorimetric or fluorometric detection than with luminescence. This anniversary year we are taking stock of all the assays and applications made possible with luminescence technologies. Check out our 30 Years and Glo-ing Celebration to learn more about how we’re celebrating luminescent assays and technologies this year.
Why you should say “Make it ‘Glo’”
Luminescence detection methods are more sensitive because they do not require excitation or filtering like fluorescence methods and do not suffer the detection limitations of colorimetric methods. A luminescent assay has a very low background signal and you simply detect all light emitted. Most luciferase enzymes have broad emission spectra, so no filters are used and light across all wavelengths is included.
Our GloMax® instruments, as well as most high-quality luminometers, contain a photomultiplier tube (PMT) that amplifies the signal allowing very low signals to be measured. Additionally, the GloMax® instrument sensitivity (gain) is automatically adjusted so it can measure both very low and very high signals on the same plate.
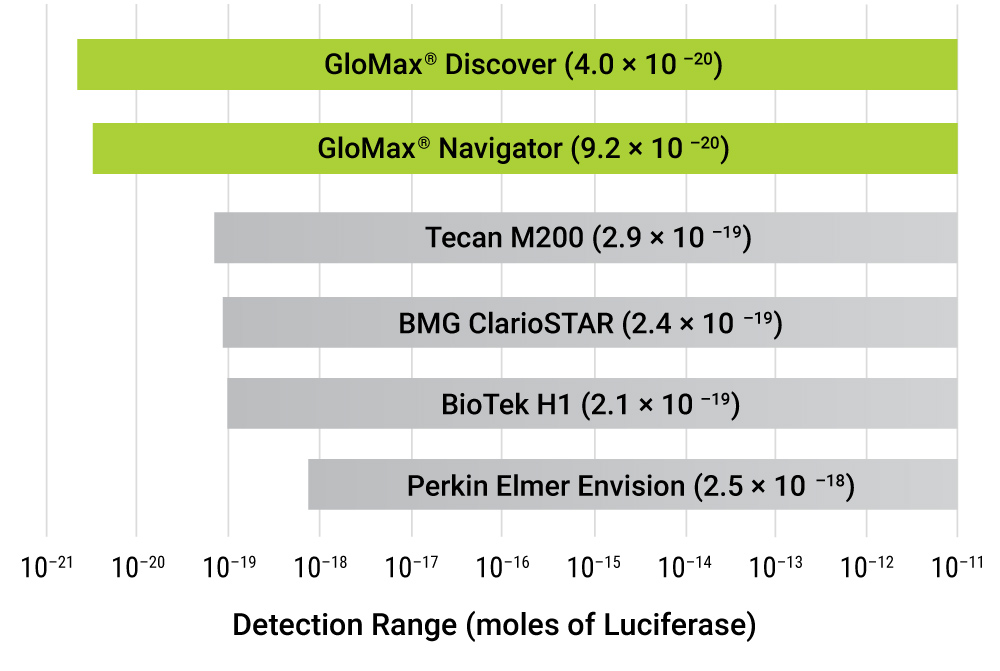
A solid white plate is best for luminescent assays because it will help reflect all the light up to the reader. Luminescence allows for detecting very small changes across a broad dynamic range of signal over 7–9 logs, depending on the instrument. This powerful tool helps to ensure that you can confidently detect a significant difference in your experiments.
Where can you “Glo”
Over the Rainbow
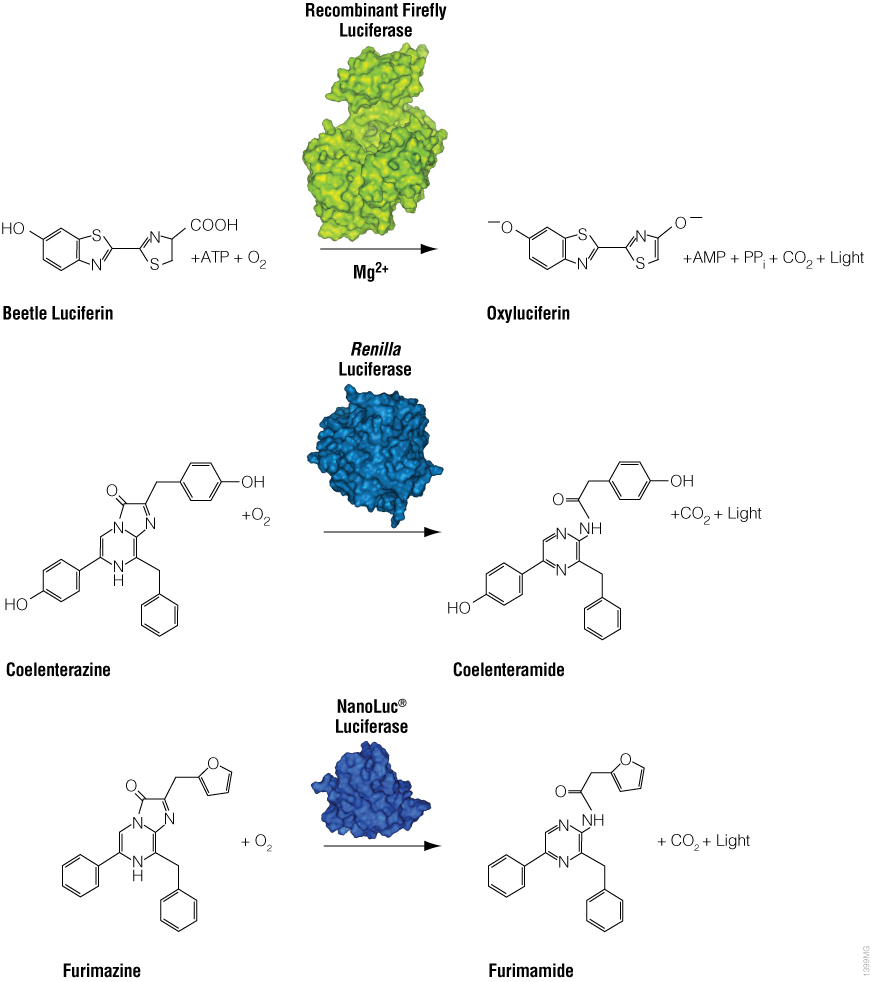
Scientists have coupled luciferase reactions to many important assay measurements in cell biology. Initially, luciferase assays were developed to measure the expression of the luciferase enzyme itself. Expression of a luciferase enzyme can be coupled to gene expression leading to a wide array of bioluminescent reporter gene assays. There is now a rainbow of luciferase reporter enzymes to choose from for different applications and for using together in dual reporter assays.
In addition to gene reporter assays, this technology is also the foundation of bioassays. These assays rely on a luciferase reporter coupled to the mechanism of action signaling pathway to measure a functional biological response for treatments like new cancer therapies.
Expression of luciferase enzymes can even used for bioluminescence imaging in live cells or whole animals and plants!
In Search of the Mysteries of Life
A luminescent signal can be coupled to more than just the luciferase enzyme itself. Assays can also use critical reaction components such as the necessary cofactors and substrates. The next family of luminescent assays use ATP as the limiting factor to generate luminescence, and the luciferase enzyme is added in excess.
In bioluminescent ATP assays, the luminescent signal depends on the amount of available ATP, so it is proportional to the number of living cells. This technology can be used to monitor cell viability and metabolism or detect microbial contamination. The foundation of ATP detection has also been coupled through other cycling enzymes to connect to a wide variety of cell signaling and energy metabolism assays.
Many cell health assays have been coupled to a modified luciferin substrate, which requires processing by the enzyme of interest before it is available for use in the luciferase reaction. If you are interested in a process that alters ATP levels or relies on the activity of a protein, then you can probably make it “Glo.”
Seeking Connections
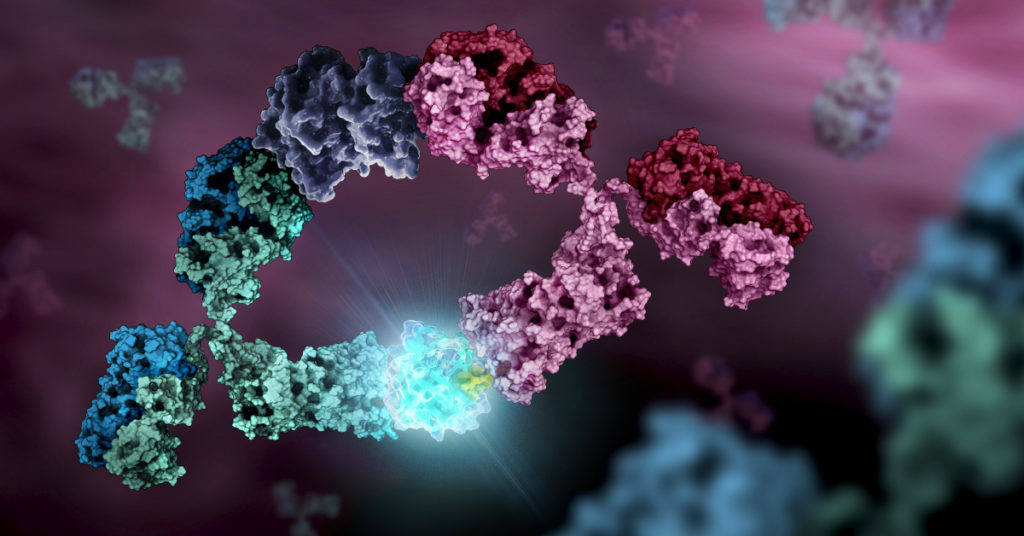
Luminescence can also work in concert with fluorescence to measure connections between proteins or molecules without an external source of excitation. Luciferase enzymes can act as an energy donor to an acceptor fluorophore in close proximity in a process called Bioluminescence Resonance Energy Transfer (BRET).
The optimized NanoBRET™ technology has allowed for increased signal and lower background to measure protein:protein interactions and also protein cellular target engagement with small molecules in living cells! The newest addition to the luminescence toolbox is the engineered NanoLuc® Binary Technology (NanoBiT®), composed of unique subunits specifically selected for low or high affinity complementation. When these subunits come together, they form a functional NanoBiT® luminescent enzyme.
The low affinity complementary pair is also useful for detecting natural protein interactions. The tiny high affinity, HiBiT®, subunit will drive the interaction with its complementary pair, so it can be used to tag proteins for quantitation. At only eleven amino acids, the HiBiT® tag is small enough for simple CRISPR–mediated tag knock-ins to examine endogenous protein expression and degradation.
Most recently, the NanoBiT® formed foundation for the simple Lumit™ Method for antigen or antibody detection. Luminescent based assays are truly connecting the pieces together to help scientists study complex processes using more biologically relevant methods than ever before.
We would love to hear from you about how luminescent assays have kept your research “Glo”-ing. You can reach the scientists in Technical Services by phone, chat, or email. If you are not sure how to get “Glo”-ing, we can even offer personalized seminars for your lab through the CoLABorate Training Program. We look forward to talking tech with you!

Joliene Lindholm is a Technical Services Scientist 3 at Promega Corporation. Joliene earned her Ph.D. in Entomology studying the molecular mode of action of an insect hormone from the University of Wisconsin-Madison before joining Promega as a Technical Services Scientist. She says, “The best thing about my job is learning about all the interesting projects that scientists are investigating; there is always something new and exciting and I get to help them succeed in their goals.”
Learn about the history of luminescence research at Promega in our recent blog, “Celebrating 30 Years of “Glo-ing” Research”
To learn more about the last 30 years of bioluminescent innovations and the discoveries they’ve enabled, please visit our 30th anniversary celebration page.
Latest posts by Joliene Lindholm (see all)
- A Day in the Life of a Technical Services Scientist - May 24, 2021
- Oh, The Ways You Can “Glo” - February 22, 2021
- Designing a Reporter Construct for Analyzing Gene Regulation - December 10, 2019

