The first monoclonal antibody (mAb) was produced in a lab 1975, and the first therapeutic mAb was introduced in the United States to prevent kidney transplant rejection in 1986. The first mAb used in cancer treatment the anti-CD20 mAb, rituximab, was used to treat non-Hodgkin’s lymphoma and chronic lymphocytic leukemia. Today therapeutic mAbs have become a mainstay of cancer, autoimmune disease, and metabolic disease therapies and include HERCEPTIN® used to treat certain forms of breast cancer, Prolia used to treat bone loss in post-menopausal women, and Stelara used to treat autoimmune diseases like psoriatic arthritis and severe Crohn disease, among many others. Therapeutic mAbs bind targets with high specificity and affinity and they can recruit effector cells to drive target elimination through mechanisms such as antibody-dependent cellular cytotoxicity (ADCC) or antibody-dependent cellular phagocytosis (ADCP), making them highly specific, effective therapies.
All approved therapeutic mAbs are IgG antibodies. IgG molecules are typically depicted as “Y-shaped” molecules consisting of two identical “fragment antigen” binding sites [F(ab)2] and one fragment crystallizable Fc domain. The F(ab)2 fragments bind the target antigen. The Fc domain is the portion of the antibody that interacts with Fcɣ receptors on effector cells such as lymphocytes and macrophages, recruiting and activating effector cells.
One way to improve mAb therapeutics is by enhancing or abolishing a particular mechanism of target elimination (ADCP or ADCC) by modulating the interactions between the Fc of the mAb and the FcɣR of the effector cells (Fc/ FcɣR interactions). A recent publication by Nath et al, describes a plate-based screening assay to measure the affinities of Fc interactions of therapeutic mAbs with six different FcɣRs. This method, which uses the NanoBiT protein complementation technology, minimizes artifacts and challenges associated with traditional biosensor platforms, and enables automated, parallel screening during therapeutic mAb development.
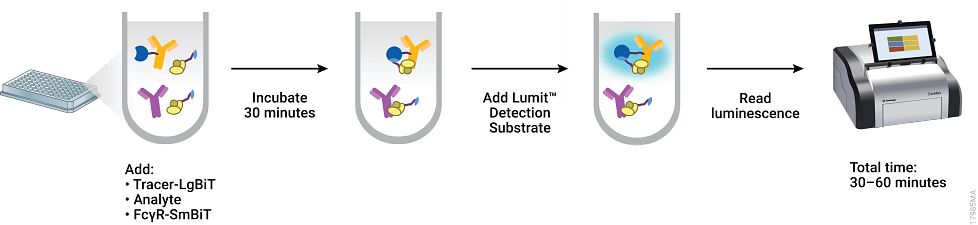
Previously the NanoBiT complementation assays have been used in applications including protein interaction studies, SARS-COV-2 antibody detection and neutralization assays, and in the development of Fc/FcRn interactions. In the work reviewed here, the authors describe an assay to measure Fc interaction with six FcɣRs: FcɣRI, FcɣRIIa (H131), FcɣRIIa (R131), FcɣRIIb, FcɣRIIIa (V158), FcɣRIIa (F158).
To fully validate the assay, the Nath et al. tested a set of 24 well characterized therapeutic monoclonal antibodies to see if the affinities obtained from the assay correspond to the those characteristic for antibody subclass of the mAbs. This test set included mAbs from IgG1, IgG2 and IgG4 subclasses, three antibody types (chimeric, humanized and human), four target types (TNFa, CD20, PD, EGFR), one Fc fusion and one NIST standard. Indeed, the NanoBiT assays data were similar to those obtained from traditional biosensor platforms and to the values expected based on the functional activity of the subclass.
The NanoBiT assay has significant advantages over traditional biosensor assays to characterize Fc/ FcɣR interactions: it can be performed with no wash steps, in multiwell plates and can be automated in a 386- or 1536-well format, allowing rapid screening of therapeutic mAbs. This parallel screening approach also allows researchers to quickly identify unique properties of mAbs, allowing a more tailored approach to antibody engineering.
Interested in learning more about this technology? Visit our Lumit FcɣR Binding Immunoassays product page.
Literature Cited
Nath, N. et al. (2022) A homogeneous bioluminescent immunoassay for parallel characterization of binding between a panel of antibodies and a family of Fcɣ receptors Sci Rep 12, 121185. https://doi.org/10.1038/s41598-022-15887-z
References
Lui, J.K.H. (2014) The history of monoclonal antibody development—Progress, remaining challenges, and future innovations. Ann. Med. Surg (Lond) 10.1016/j.amsu.2014.09.001
Manis, J. P. 2022 Overview of therapeutic monoclonal antibodies. UpToDate https://www.uptodate.com/contents/overview-of-therapeutic-monoclonal-antibodies#H1
Pierpont, T. M. et al. (2018) Past Present and Future of Rituximab—The World’s First Oncology Monoclonal Antibody Therapy. Frontiers in Oncology https://doi.org/10.3389/fonc.2018.00163
Michele Arduengo
Latest posts by Michele Arduengo (see all)
- The Casual Catalyst: Science Conversations and Cafes - July 18, 2024
- Cancer Moonshot: Solving Tough Problems - May 28, 2024
- Automated Sampling and Detection of ToBRFV: An Emerging Tomato Virus - April 25, 2024
