Updated 02/12/2021
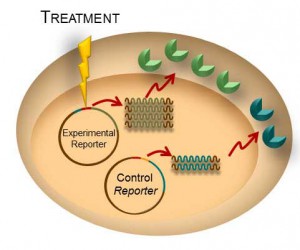
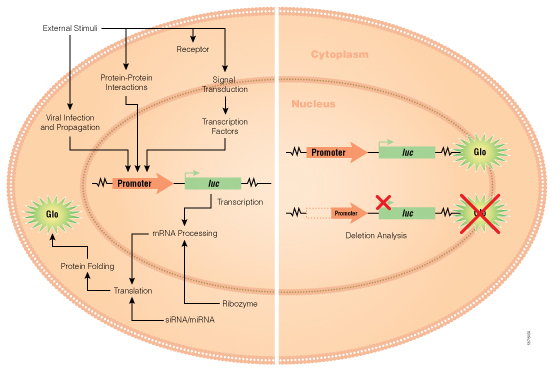
Transient transfection is often used to perform reporter assays. We have advocated using a dual-reporter system for decades to normalize the data obtained and gain a clearer understanding of your results. The experimental reporter should vary with treatment and the control reporter should vary little with treatment. The control reporter thus serves as a marker to help you understand the relative activity of your experimental reporter. The bioluminescent Dual-Luciferase® method allows for sequential detection of the second reporter in a single sample providing a simple two-step normalization method. Here are seven ways in which dual-reporter assays help you avoid misinterpreting results.
Simply comparing the ratio of the experimental to the control reporter can resolve differences in:
- Number of Cells/Well: When manually pipeting cells into a 96-well plate, there is always a chance of having variable numbers of cells in each well. This variation is cell number will affect the experimental and control reporters equally, so the ratio of experimental:control reporter activity will eliminate false interpretation of the experimental data–whether it affects an entire row or column on the plate or individual wells.
- Transfection Efficiency: The variations in transfection efficiency will equally affect both the experimental and control reporters so the ratio of activity in dual-reporter assays will normalize the data.
- Cell Viability: Often, reporter assays look at the dose response curve of a particular compound with regard to gene expression. Ideally, if a compound causes a change in the experimental reporter the control reporter will demonstrate little effect. However, if the compound is toxic, both the experimental and control will be altered and the ratio will tell you whether the compound truly affects reporter activity or just kills the cells.
- Lysis Efficiency: When lysing a plate of cells, you could encounter situations where rows or columns lyse differently, especially if you are using manual disruption or get interrupted mid-plate. The difference is lysis will affect the experimental and control equally so the ratio will remove the variation.
- Temperature: Ideally, a plate should be equilibrated to ambient room temperature before proceeding to the reporter assay. Plates can cool at different rates or researchers anxious to record data may read the data early. Temperature variations will affect both reporters so the ratio will limit the affect on the data.
- Measurement Time: Repetition of data is a hallmark of good science. You are often called upon to repeat experiments sometimes days or weeks apart. Let’s say you repeat your experiment one week after the initial experiment. The first time you measured the response, you waited 10 minutes after reagent addition to read, this week you waited 30 minutes. This will affect both reporters equally and therefore the ratio will allow you to more easily compare the data from this week and last week.
Bonus Benefit from Dual-Luciferase®, Dual-Glo® and the NanoGlo® Dual Luciferase Reporter Systems: No Lysate Splitting: Promega dual-reporter assays are designed for same-well multiplexing so there is no chance of variations creeping into your data due to unequal splitting of the cellular lysate to measure two separate reporter activities.
Since the introduction of the first bioluminescent dual-luciferase assay in 1995, this approach has been used in countless studies to advance our scientific understanding of cellular gene regulation.