It’s been just over 10 years since the world lost a pioneering immunologist and biochemist, Dr. Jürg Tschopp. He died tragically during a hiking trip in the Swiss Alps on March 22, 2011. A host of academic journals, including Science, Nature and Cell, paid tribute to Dr. Tschopp with obituaries that highlighted his many accomplishments in the fields of apoptosis and immunology.
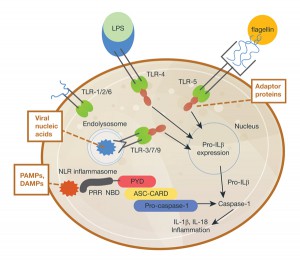
In 2002, a team led by Dr. Tschopp at the University of Lausanne, Switzerland, was studying the role of the proinflammatory cytokine interleukin 1 beta (IL-1β). This cytokine is produced in the cytoplasm as an inactive precursor (pro-IL-1β). It is cleaved by caspase-1 to the active form, but the exact process by which caspase-1 itself is activated was unknown at the time. Several members of the caspase family contain a conserved region known as the caspase recruitment domain or CARD, and it was proposed that this domain was essential to caspase activation.
Based on similarity to another protein containing an N-terminal CARD motif (Apaf-1) that is involved in activation of caspase-9, the researchers examined the roles of a family of proteins known as NALP1, NALP2 and NALP3 (1). In particular, they were interested in NALP1, which is involved in the immune response. Unlike Apaf-1, NALP1 contains a CARD motif at the C terminus, while the N terminus contains a related motif known as a pyrin-like domain (PYD). The research team had previously showed that the PYD region of NALP1 interacted with an adapter protein known as PYCARD or ASC, which also contains an N-terminal PYD and C-terminal CARD.
The results of the team’s in vitro binding, activation and immunodetection studies showed that a multi-unit protein complex is responsible for caspase activation, and they called this complex the “inflammasome” (1). It is composed of caspase-1, caspase-5, PYCARD/ASC and NALP1.
Continue reading “The NLRP3 Inflammasome: Flipping the Switch”