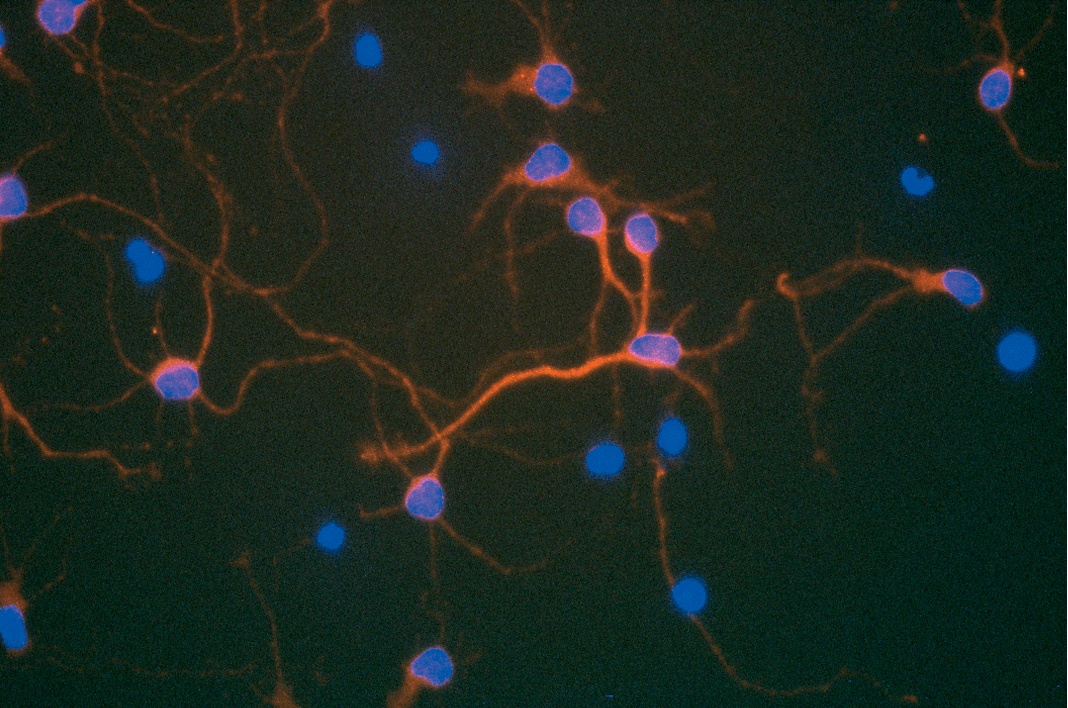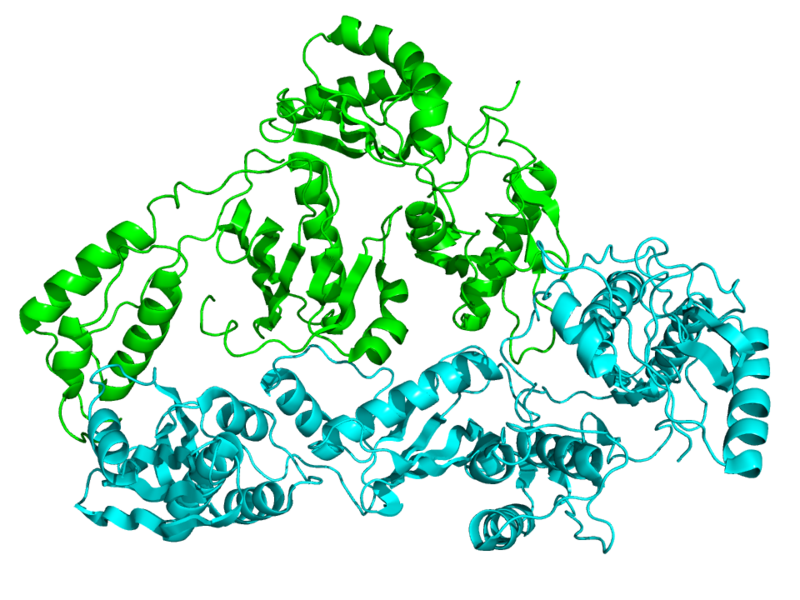
Today, reverse transcriptases are commonplace molecular biology tools, easy to obtain and routinely used in labs for everyday cloning and gene expression analysis experiments. Reverse transcriptase inhibitors have also found widespread use as antiviral drugs in the treatment of retroviral infections.
It’s easy to forget that the existence of reverse transcriptase activity—the ability to convert an RNA template into DNA—was once a revolutionary notion not easily accepted by the scientific community. The idea that RNA could be the template for DNA synthesis challenged the “DNA–>RNA–> Protein” central dogma of molecular biology.
The foundational studies that proved the existence of a reverse transcriptase activity in RNA tumor viruses were described in two papers published back-to-back in Nature in June, 1970. Two of the authors of these studies, Howard Temin of the University of Wisconsin and David Baltimore of the Massachusetts Institute of Technology, were awarded a Nobel Prize for their work in 1975.
In appreciation of the significance of these papers, the editorial introduction published in Nature at the time states:
This discovery, if upheld, will have important implications not only for carcinogenesis by RNA viruses but also for the general understanding of genetic transcription: apparently the classical process of information transfer from DNA to RNA can be inverted.
Before these papers were published, it was known that successful infection of cells by RNA tumor viruses required DNA synthesis. Formation of virions could be inhibited by Actinomycin D—an inhibitor of DNA-dependent RNA polymerase—so it was known that synthesis of viral RNA from a DNA template was part of the viral life cycle. The existence of an intracellular DNA viral genome was therefore indicated, and had been postulated by Temin in the mid 1960’s. However, proof of the mechanism whereby this DNA template was generated from the RNA genome of the infecting virus remained elusive. Continue reading “Elegant Experiments that Changed the World”
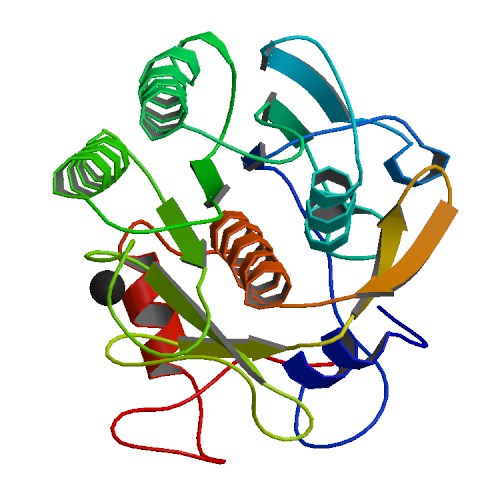

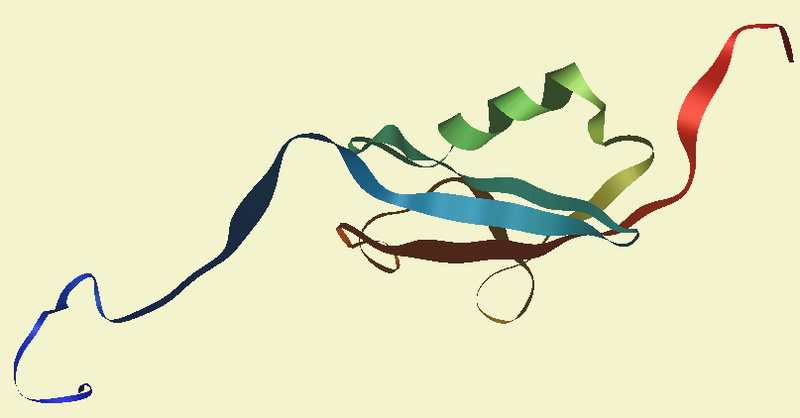
 The ability to manipulate genes and proteins and observe the effects of specific changes is a foundational aspect of molecular biology. From the first site-directed mutagenesis systems to the development of knockout mice and RNA interference, technologies for making targeted changes to specific proteins to eliminate their expression or alter their function have made tremendous contributions to scientific discovery.
The ability to manipulate genes and proteins and observe the effects of specific changes is a foundational aspect of molecular biology. From the first site-directed mutagenesis systems to the development of knockout mice and RNA interference, technologies for making targeted changes to specific proteins to eliminate their expression or alter their function have made tremendous contributions to scientific discovery.