On May 21st, 2021 we celebrate National Endangered Species Day. This day helps raise awareness and increase knowledge of endangered species and wildlife, in hopes to save them. We have been lucky enough to collaborate with organizations and partners to help save species that were on the brink of extinction. Take a look at some species that are hoping for a second chance to survive and thrive.
Kit Elizabeth Ann the Black-Footed Ferret

In February 2018, resurrection efforts began for the then endangered black-footed ferret. With the help of the U.S. Fish and Wildlife Service, Revive and Restore, partners ViaGen Pets & Equine, San Diego Zoo Global, and the Association of Zoos and Aquariums, the successful cloning of a black-footed ferret was announced in February 2021. “Elizabeth Ann” was cloned from Willa, a female ferret that died in 1988, using somatic cell nuclear transfer (SCNT). Elizabeth Ann’s genetic variants reveal a lot of much-needed hope for the genetic diversity of wild ferrets. Check out the full story on Elizabeth Ann’s journey here!
Continue reading “Shifting Conservation Status: Endangered Species Get a Second Chance”

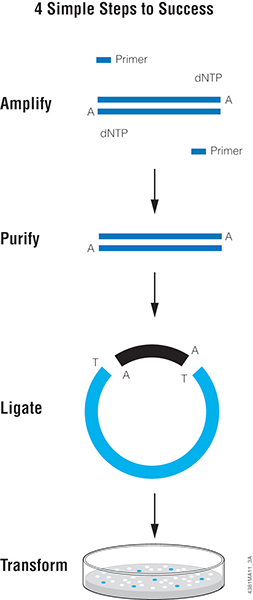
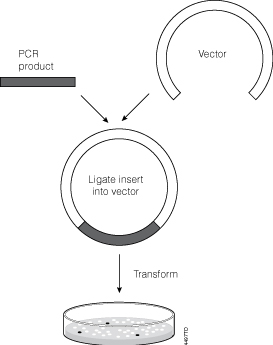
 Let’s face it, most lab techs and purchasing agents aren’t all that happy when you send them an Instagram picture of your latest lunchroom-napkin cloning strategy as your order form for your next big cloning experiment. So we have created the
Let’s face it, most lab techs and purchasing agents aren’t all that happy when you send them an Instagram picture of your latest lunchroom-napkin cloning strategy as your order form for your next big cloning experiment. So we have created the  Already have a favorite vector and a freezer full of restriction enzymes? No problem, skip those steps and move on to getting the perfectly sized nucleic acid markers or the particular polymerase your experiment requires.
Already have a favorite vector and a freezer full of restriction enzymes? No problem, skip those steps and move on to getting the perfectly sized nucleic acid markers or the particular polymerase your experiment requires.