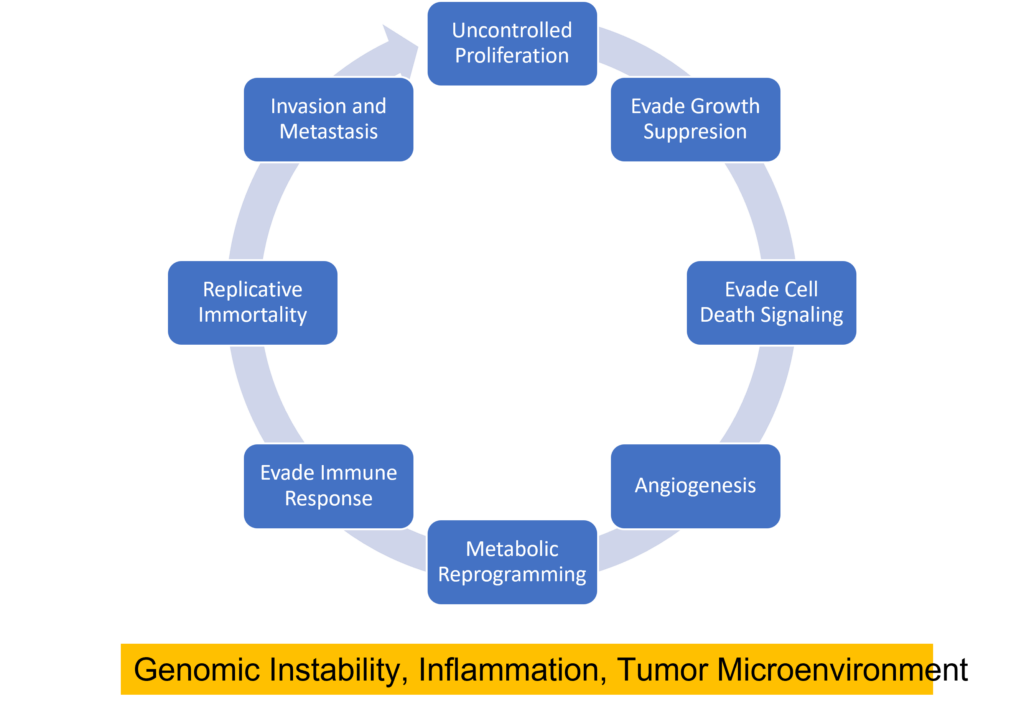
Cancer cells can be distinguished from normal cells by a variety of features including their ability to inappropriately activate signals for cell proliferation, evade growth suppression from contact inhibition or tumor suppressor activity, evade cell death signals, replicate DNA continually, induce angiogenesis, invade new tissues, reprogram their metabolism to provide energy for rapid proliferation, and evade immune detection (1) . Several biological processes are responsible for these features including genomic instability, inflammation, and the creation of a tumor microenvironment.
The tumor microenvironment is the network of non-malignant cells, connective tissue and blood vessels that surround and infiltrate the tumor. These surrounding “normal” cells interact with each other and the cancer cells and are important players in tumorigenesis. One cell type that is often found in the tumor microenvironment are nerve cells. In fact, cancer cells often express proteins that encourage nerve growth into the tumor microenvironment such as growth factors and axon-guidance molecules (2). Crosstalk between nerve cells and tumor cells can facilitate development of several cancer types (2) including pancreatic, head and neck, oral, prostate, and colorectal cancers.
Recent studies have investigated the nerve crosstalk between oral cancers and nerve cells. Amit and colleagues published a paper in 2020 looking at crosstalk between oral squamous cell carcinoma cells and neurons in the tumor microenvironment (3). Looking at data from the Cancer Genome Analysis they found a pattern indicating that oral tumors with many nerves in the tumor and its tumor microenvironment coupled with mutations in TP53, a tumor suppressor gene, were associated with earlier death. Using mouse models, they were able to show a causal relationship between TP53 mutations and increased nerve density. In additional experiments they were able to show that the tumors were sending “growth signals” to neurons using microRNAs secreted in extracellular vesicles (EVs). The microRNAs in these EVs resulted in reduction of normal neuronal activity and the “reprogramming” of the neural cells from a sensory neuron to more of an adrenergic neuron. Adrenergic signaling is associated with promoting tumor growth and progression, and it may drive resistance to resistance to chemotherapies (2). Injecting wildtype p53 into tumors suppressed this reprogramming, and blocking adrenergic signaling resulted in slower-growing tumors.
A second study, published in Cell Metabolism by Zhang and colleagues (4), showed that the nerve cells recruited to the tumor microenvironment of oral cancers help the tumor survive by producing signaling molecules that allow the cancer cells to continue to grow and proliferate, even in a nutrient depleted environment. The researchers noted that patients with oral squamous cell carcinoma (OSCC) often report severe pain, and there are many nerves in and around the tumors. Furthermore, uncontrolled pain was often associated with poorer outcomes. In cell culture experiments, the team discovered that communication between the tumor and nerves relied upon a nutrient depleted microenvironment. In fact, feeding sugar water to the mice with OSCC slowed tumor growth. The tumor associated nerves in mice secreted calcitonin gene-related peptides (CGRP). These molecules are associated with pain pathways and wound healing. Looking at CGRP levels in tissue samples from individuals with OSCC, the researchers noted that higher levels of CGRP were correlated with poorer clinical outcomes.
To see if blocking CGRP signaling could improve outcomes, the researchers turned again to the mouse models, and found that the CGRP blocking drug rimegepant rendered the tumors susceptible to starvation, inhibiting their growth. Interestingly rimegepant is one member of a class of drugs used to treat migraines and is already approved for use in humans.
A better understanding of nerve-cancer crosstalk will provide many new therapeutic targets for cancer therapies. Existing, approved neuromodulatory therapeutics may well become an important part of the strategy to overcome cancer drug resistance and new therapies that specifically target the neural recruitment and reprogramming in the cancer microenvironment may hold promise as new ways to approach cancer therapy.
Literature Cited
- Hanahan, D. and Weinberg, R. A. (2011) Hallmarks of cancer: the next generation. Cell 144:646–74. DOI: 10.1016/j.cell.2011.02.013
- Silverman, D. A. et al. (2021) Cancer-Associated Neurogenesis and Nerve-Cancer Cross-talk Cancer Research 81:1431-40 https://doi.org/10.1158/0008-5472.CAN-20-2793
- Amit, M. et al. (2020) Loss of p53 drives neuron reprogramming in head and neck cancer. Nature, 578: 449–54. https://doi.org/10.1038/s41586-020-1996-3
- Zhang, Yu. et al. (2022) Cancer Cells Co-opt Nociceptive Nerves to Thrive in Nutrient-Poor Environments and Upon Nutrient-Starvation Therapies. Cell Metabolism 34:1999–17. https://pubmed.ncbi.nlm.nih.gov/36395769/
Michele Arduengo
Latest posts by Michele Arduengo (see all)
- The Casual Catalyst: Science Conversations and Cafes - July 18, 2024
- Cancer Moonshot: Solving Tough Problems - May 28, 2024
- Automated Sampling and Detection of ToBRFV: An Emerging Tomato Virus - April 25, 2024
