In the fifty years since the first reported transformation of recombinant plasmids into bacteria (1), plasmid cloning has become one of the pillars of synthetic biology research and manufacturing biopharmaceuticals.
But purifying plasmids is no small feat. It can often take hours of hands-on time to go from culture to eluate with low-throughput and time-sensitive manual methods. Automating plasmid purification is the way to go, whether you’re isolating a single plasmid from a large volume culture or creating a library of thousands of different constructs.

If you need a detailed refresher on plasmid DNA purification and how our automated isolation kits can help you get more consistent plasmid yields and purity, check out our DNA purification guide.
How Is Purification Automated for Plasmids?
The classic approach to plasmid purification is a process called alkaline lysis (2). In general,
- After bacteria are isolated from culture via pelleting and resuspended, the cells are lysed with an alkaline solution of sodium hydroxide and SDS (pH 12.0-12.5). This step breaks down bacteria cell membranes and denatures nucleic acids. It is the main difference between plasmid purification and other nucleic acid purification methods.
- The pH of the lysate is neutralized using acetate, causing proteins, RNA and chromosomal DNA to precipitate. In contrast, circular DNA renatures properly and remains soluble.
- Centrifuging (or filtering) the sample provides a supernatant containing plasmid, salts, debris and other soluble species.
- The plasmid is then, generally, purified by precipitation and resuspension, binding to silica beads or a spin column, or ion-exchange chromatography.
One challenge while carrying out this method is that you need to be very gentle with the sample during the lysis step, otherwise nuclear DNA can shear and will not precipitate out of solution during neutralization, contaminating your final eluate. Also, if the alkaline lysis step takes too long, the circular DNA may remain permanently denatured and will not reform the desired plasmids.
Automated plasmid purification approaches follow similar steps but largely avoid centrifugation steps (after initial pelleting of the bacterial cells). Instead, the plasmid is typically captured on magnetic silica beads, which can be manipulated by an automated system and exposed to different washing and elution steps. Some methods, like those that use our Wizard® MagneSil ® Tfx System, will also include steps specifically designed to remove endotoxins.
Watch this webinar on-demand to learn more about how automating plasmid purification streamlines drug development.
A well-designed automated purification system can increase throughput and save you hands-on time. Automation can increase consistency across your plasmid purifications.
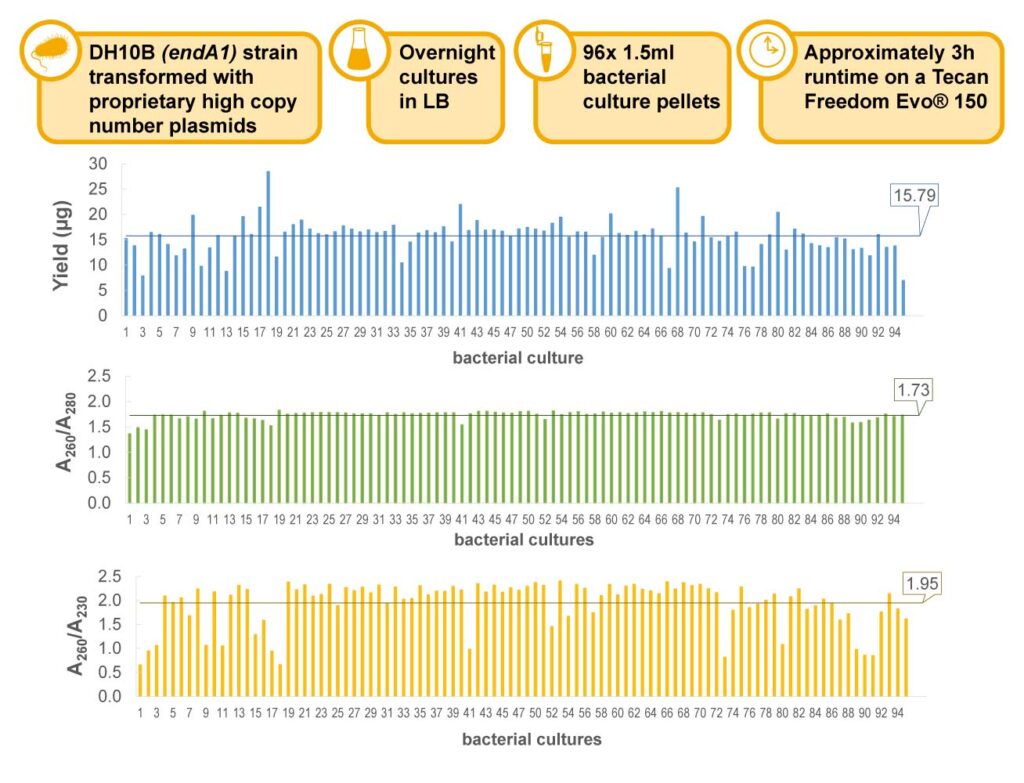
How do Our High-Throughput Plasmid Purification Kits Differ?
We offer two different kits for high-throughput, automated purification of plasmids:
- Wizard® MagneSil ® Tfx System
- Wizard® MagneSil® Plasmid Purification System
Both kits are designed to handle plasmid purification from 0.5–200ml of bacterial culture and can be completely automated—and there are no centrifugation steps. Each requires about one hour of processing time per plate and is compatible with a range of different automation systems, including liquid handlers from Hamilton, Tecan and Beckman Coulter.
There is a key difference, however. Compared to the Wizard® MagneSil® Plasmid Purification System, the Wizard® MagneSil® Tfx System includes an endotoxin removal step, and so is ideal if transfection is one possible application of your plasmid purification workflow.
In contrast, the Wizard® MagneSil® Plasmid Purification System does not include endotoxin removal and its primary applications are cloning, PCR and sequencing. If you plan to use your isolated plasmids for transfection, then the Wizard® MagneSil® Tfx System is a better choice. Otherwise, the Wizard® MagneSil® Plasmid Purification System should meet your needs.
Download this research poster about using the Wizard® MagneSil® Tfx System for plasmid purification in an engineered antibody screening workflow.
One area in which these kits have been used successfully is in university core labs that focus on high-throughput production of recombinant proteins for research use. These labs consistently find that the Wizard® MagneSil® kits provide higher and more consistent yields than competing systems.
Read how the 2019 iGEM Marburg team worked with our Field Support Scientist team to automate plasmid purification.
Another major advantage of using Promega automated plasmid purification chemistries is access to our Field Support Scientists who can design and optimize automated workflows, write scripts for liquid handler systems and provide other on-site support options.
Find the lab automation resources you need at Promega
References:
- Cohen, S.N. et al. (1973) Construction of biologically functional bacterial plasmids in vitro. PNAS 70, 3240.
- Birnboim, H.C. and Doly, J. (1979) A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7, 1513.
Latest posts by Jordan Nutting (see all)
- The Central Dogma of Promega: The Story and Science Behind Our Kit Packaging Design - May 7, 2024
- Silencing the Immunogenicity of AAV Vectors - April 4, 2024
- Discovering Cyclic Peptides with a “One-Pot” Synthesis and Screening Method - February 29, 2024
