Large-scale analyses of the proteome have revealed proteomic changes in response to disease, and these changes hold great promise for diagnostics and treatment of complex diseases if proteomic analysis can be brought into the clinical laboratory. Successful and reliable large-scale proteomics requires sample preparation workflows that are reproducible, reliable and show little variability. To bring proteomics into the clinical laboratory, standardized procedures and workflows for sample prep and analysis are required to generate valid, actionable results on a time scale useful for the clinic.
The two most common sample types analyzed for clinical proteomics are body fluids and tissue biopsies. To process these kinds of samples, there are two initial steps: tissue solubilization, followed by proteolytic digestion. Solubilization of solid tissues is the most labor-intensive and produces the most variable results.
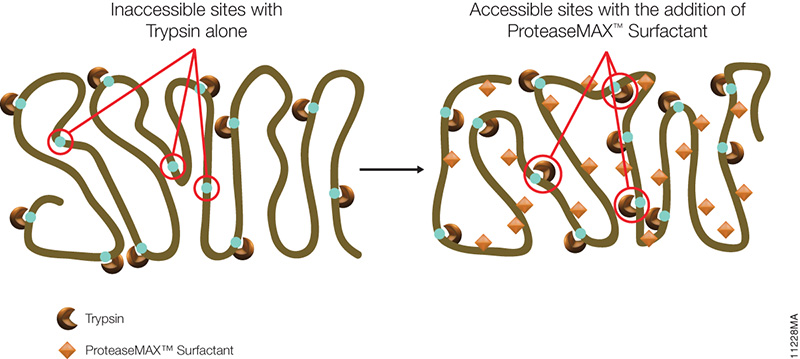
The introduction of pressure cycling technology (PCT) using Barocycler instrumentation has greatly improved both tissue solubilization and digestion consistency. The PCT-based sample preparation protocols generally utilize urea as a lysis buffer for protein denaturing and solubilization. Urea has several drawbacks including inhibiting trypsin activity and introducing unwanted modifications like carbamylation.
Lucas and colleagues analyzed whether replacing urea with SDC would produce similar tissue digestion profiles and improve the PCT method.
Are you looking for proteases to use in your research?
Explore our portfolio of proteases today.
SDC allowed the use of higher temperatures compared to urea, and hence the first step (lysis, reduction, and alkylation) was performed at 56 °C. The second digestion step in the Barocycler was optimized, and the third step was eliminated. To further reduce digestion time, they capitalized on Rapid Trypsin/Lys-C. Rapid Trypsin/Lys-C maintains robust activity at 70 °C, and allowed Barocycler digestion to be performed in a single step, completing digestion in 30 cycles (approximately 30 min) rather than 105 minutes, streamlining the protocol.
The data presented an improved conventional tissue PCT approach in a Barocycler by replacing urea and proteolytic enzymes with SDC, N-propanol, and modified commercially available enzymes that have higher optimum temperatures.
Try a sample of high-efficiency Trypsin Platinum today!
Visit our website for more on Trypsin Platinum, Mass Spectrometry Grade, with enhanced proteolytic efficiency and superior autoproteolytic resistance.
Paper Referenced
Lucas, N. et al. (2019) Accelerated Barocycler Lysis and Extraction Sample Preparation for Clinical Proteomics by Mass Spectrometry. J of Proteome Res 18, 399–405.
Related Posts
Like this:
Like Loading...