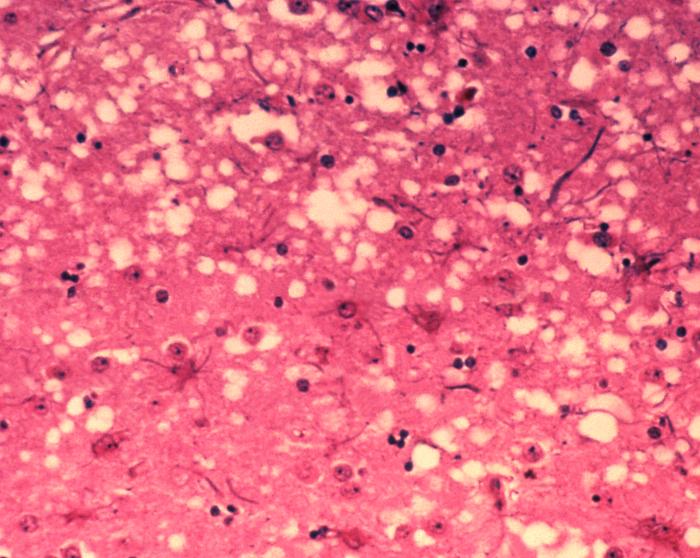
In the late-80’s through the 90’s, food and health agencies focused on a mysterious fatal brain disease that infected thousands of cattle. Bovine spongiform encephalitis—or “mad cow disease”—is caused by an infectious protein called a prion. Despite fears that tainted meat would cause the disease to spread to humans, mad cow disease never really made an impact on human health. However, forms of the prion disease such as Creutzfeldt-Jakob disease do affect humans.
In addition to Creutzfeldt-Jakob disease, many neurodegenerative diseases such as Alzheimer’s, Parkinson’s, Huntington’s and amyotrophic lateral sclerosis (ALS or Lou Gehrig’s disease) are now thought to be a result of prion-like activity. There is no cure for these diseases, however, new experimental treatment strategies might help slow the progression of neural degeneration.
 Back in 2015 the Ice Bucket Challenge brought Amyotrophic Lateral Sclerosis (ALS) to public attention, initiating worldwide pleas for more funding of research toward a cure for this fatal disease, which is characterized by progressive degeneration of motor neurons. In spite of many efforts over the last few decades, the precise cause of ALS is still unknown.
Back in 2015 the Ice Bucket Challenge brought Amyotrophic Lateral Sclerosis (ALS) to public attention, initiating worldwide pleas for more funding of research toward a cure for this fatal disease, which is characterized by progressive degeneration of motor neurons. In spite of many efforts over the last few decades, the precise cause of ALS is still unknown.