Attention-Deficit/Hyperactivity Disorder (ADHD) is a complex neurodevelopmental disorder that affects millions worldwide. Current therapeutic treatment relies on pharmaceutical approaches, but emerging research suggests that dietary supplements, such as omega-3 fatty acids, may offer complementary therapeutic options. A recent study published in the Journal of Psychiatric Research explores the relationship between inflammation and dietary supplements to determine how they might influence ADHD pathology. This work was conducted in Dr. Edna Grünblatt’s lab at the University of Zurich and was supported through Promega’s Academic Access Program. I had the chance to interview Dr. Natalie Walter, the lead author, to learn more about how her work offers potential opportunities for non-pharmacological interventions.
The Research Focus
While stimulant medications like methylphenidate are commonly prescribed to manage ADHD symptoms, there is growing interest in complementary strategies that might reduce reliance on pharmaceuticals or enhance their effects. Natalie’s project centered around a timely question: can omega-3 fatty acids serve as an effective, non-pharmacological treatment for ADHD? With ADHD’s multifactorial origins—spanning genetic, environmental, and inflammatory components—her team was particularly interested in the role of pro-inflammatory cytokines, specifically IL-6 and TNF-α. Alternations in pro-inflammatory cytokines have been found in the blood-levels of ADHD patients, with prior studies demonstrating their dynamics playing a crucial role in regulation proliferation, differentiation, and synaptic maturity in nervous system cells.
“ADHD is so broad… there are environmental factors and genetic factors… and inflammation came up a lot of times—association to inflammation, ADHD—so we wanted to see if inflammation such as cytokine release of IL-6 and TNF-α will have an effect in ADHD.”
The primary pharmaceutical treatment for ADHD is the Methylphenidate. Despite high response rates, adverse side effects and high variability between patients has led to a demand for additional treatment options. Omega-3 fatty acids have been proposed as a potential supplementation for conditions that display dysregulated inflammatory mechanisms due to their antioxidant and anti-inflammatory regulation. Natalie sought out to observe how omega-3s might modulate the inflammatory response and contribute to treatment potentials in ADHD patients.
A Novel Model System
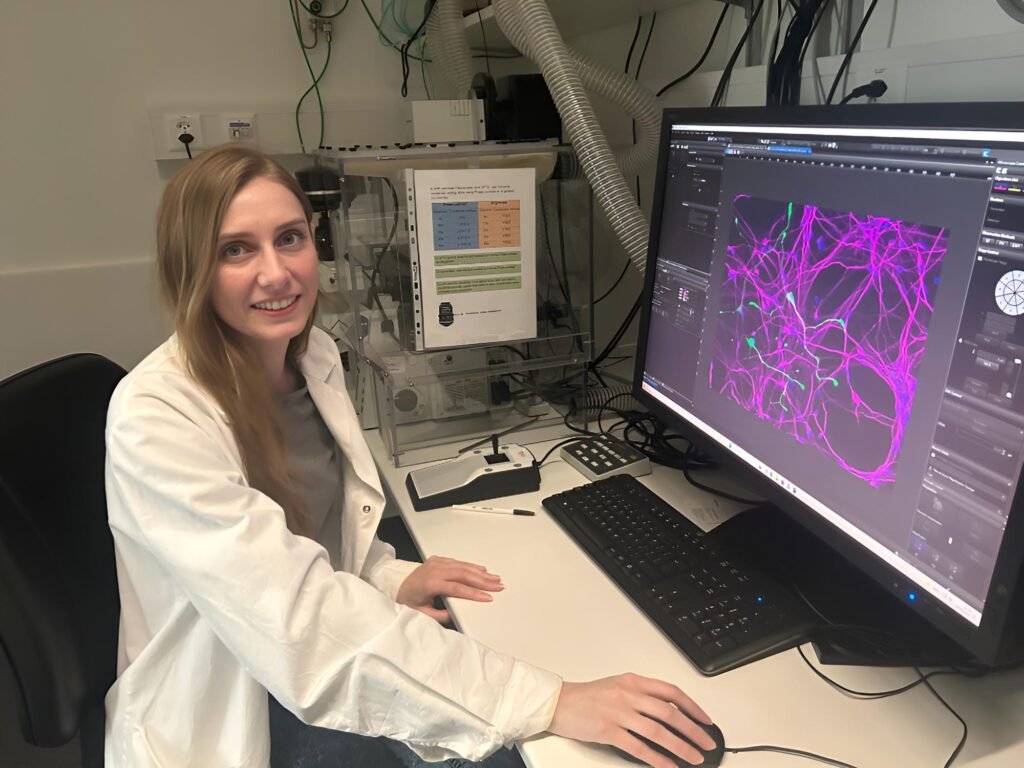
To explore this hypothesis, Natalie’s lab used induced pluripotent stem cells (iPSCs) reprogrammed from ADHD patients’ blood or hair follicles from the hospital affiliated with The University of Zurich. These were differentiated into neurons, creating a highly relevant in vitro model for the disorder. This cutting-edge approach enabled the team to study cellular responses in a patient-specific context—an important step toward personalized medicine.
“What was special about our lab specifically is also that we used induced pluripotent stem cells which were than differentiated into neurons… retrieved from the actual patient… so we could have a better representation of the effects.”
While working with iPSCs required considerable patience and precision, the insights gained from this model provided a strong foundation for translational research.
“It’s really an upcoming [area] and might really help also in regenerative medicine, but not only that—also personalized medicine.”
Characterizing Inflammation using Promega Lumit® Assays
This research looked closely at the relationship of neuroinflammation and oxidative stress. To measure cytokine expression as part of her study, Natalie looked for assays that would integrate easily with the techniques her lab was already using. Based on prior experience with Promega’s luminescent reporter assays, she decided to try Promega’s Lumit® cytokine assays for IL-6 and TNF-α. The assay’s no-wash, luminescent format aligned well with her existing equipment and protocols, making it a practical addition to her experimental workflow.
“It worked really well… very user friendly.”
The Lumit® cytokine assays enabled Natalie to track changes in IL-6 and TNF-α levels with clarity and reproducibility. In her experiments, untreated ADHD patient-derived neurons (FCNs) exhibited reduced TNF-α secretion and normal IL-6 secretion when compared to controls. Treatment with an omega-3 fatty acid, Arachidonic acid (AA), led toa measurable increase in TNF-α from the control cells, likely due to the pro-inflammatory response typically expected from AA. This response did not occur in the ADHD FCNs. Lower levels of TNF-α could indicate a lower reactivity to inflammation and stress, potentially resulting to long-term difficulties for cellular protection. Her findings align with a growing body of research suggesting that inflammation may play a key role in ADHD’s development and severity.
Beyond these inflammatory responses, increased superoxide levels present in the ADHD FCNs were reduced upon treatment with Eicosapentaenoic acid (EPA), further demonstrating the potential of omega-3 fatty acids in alleviating abnormal characteristics of ADHD cells. These findings supported the hypothesis that omega-3 fatty acids offer potential as a complementary treatment for ADHD. Methylphenidate remains an effective first-line treatment for many individuals, however research exploring dietary interventions could benefit patients and caregivers seeking complementary holistic treatment options.
Broader Implications
Natalie’s work touches on themes central to the future of healthcare: personalized medicine and translational neuroscience. By using patient-derived cells and clinically relevant biomarkers, her research paves the way for more targeted, individualized treatment plans.
“With the research, we hope that it’s not just testing like trial and error, but that we can then somehow find evidence to say, ‘OK, for your specific child, this treatment is better due to this research.'”
She also envisions future studies using blood or saliva samples to further investigate cytokine levels as potential biomarkers. Such tools could help clinicians identify which patients may respond best to omega-3 supplementation, reducing the current trial-and-error approach.
What’s Next
Although Natalie has since completed her PhD, her lab continues exploring inflammation-related pathways in ADHD using cell culture systems. As for Natalie, she’s interested in continuing to bridge the gap between science and society and making complex data more accessible and trustworthy.
This research exemplifies how thoughtful experimental design, innovative model systems, and robust tools like Promega’s Lumit® cytokine assays can come together to push the boundaries of neuroscience. Her work highlights not only a promising path for ADHD treatment but also the importance of accurate, reproducible tools in modern research.
Read the full publication: Investigating the impact of omega-3 fatty acids on oxidative stress and pro-inflammatory cytokine release in iPSC-derived forebrain cortical neurons from ADHD patients – ScienceDirect
Learn more about our Inflammation Assays.
Learn more about Promega’s Academic Access Program.
Latest posts by Simon Moe (see all)
- Insights from 3D Liver Models: Rethinking Fatty Liver Disease with Hormone Correction - October 29, 2025
- Top 5 Luciferase Reporter Vectors You Didn’t Know You Needed (But Now Can’t Live Without) - October 21, 2025
- Non-Pharmacological Approaches to ADHD: Exploring Inflammation and Omega-3s - May 22, 2025
