This blog was written by guest author Michael Curtin, Senior Product Manager, Small Molecule Drug Discovery.
ATP is the universal energy currency of cells, and thousands of proteins outside the kinase family “spend” it to move cargo, remodel nucleic acids, pump ions, or fold proteins. These ATP-hydrolyzing enzymes—collectively known as ATPases—span functional classes including motor proteins, transporters, chaperones, chromatin remodelers, ligases, and, crucially for genome stability, helicases.
From DNA replication to RNA processing, helicases are essential players. DNA/RNA helicases such as MCM, XPB/XPD, WRN, and members of the DDX family sit alongside AAA+ unfoldases, ABC transporters, and V-ATPases—all drawing on ATP to power their molecular work.
Why Focus on Helicases?
Unlike chaperones or pumps, helicases couple ATP hydrolysis directly to mechanical work on nucleic acids—unwinding duplexes, displacing bound proteins and orchestrating dynamic repair complexes. This tight coupling means ATP turnover directly reflects enzymatic activity, making helicases ideal candidates for mix-and-measure luminescent assays like the ADP-Glo™ Max Assay.
Because each strand translocation cycle releases stoichiometric ADP, helicases rank among the most robust enzyme classes for ADP-Glo™ screening. They’re also increasingly relevant in therapeutic discovery, especially in the context of tumor-selective vulnerabilities.
WRN: A Target in MSI-High Tumors
WRN, a RecQ-family helicase, has become a compelling target in microsatellite instability-high (MSI-H) cancers. Functional genomics screens have shown that MSI-H tumors are critically dependent on WRN function, establishing its ATPase activity as a druggable liability.
The ADP-Glo™ Assay has enabled direct comparison of lead inhibitors like Novartis’ HRO761 with next-generation analogs. In a 2025 medicinal chemistry campaign, Moon et al. used ADP-Glo to identify a benzimidazole analog (10a) that reduced the IC₅₀ for WRN ATPase inhibition from 88nM to 5nM, outperforming ten other scaffolds in vitro (1). However, potency improvements did not always yield stronger antiproliferative effects in MSI-H cells, underscoring the need for rapid biochemical screening before cell-based validation.
Why it matters: WRN inhibitors, such as HRO761 and KWR095, collapse replication forks in MSI-H colorectal and endometrial cancer cells while sparing normal tissue. ATP hydrolysis underlies this replication fork protection, making ADP-Glo™ Assays a key tool for both high-throughput screening and mechanistic profiling (1).
Pol θ: A Dual-Function Polymerase in HR-Deficient Tumors
Pol θ is an A-family polymerase fused to a helicase-like ATPase domain. HR-deficient tumors—such as BRCA1/2 mutants—depend on Pol θ-mediated end joining, making its ATPase activity a viable therapeutic target.
Novobiocin (NVB), a first-in-class allosteric Pol θ ATPase inhibitor, was characterized using 14-point
ADP-Glo™ Assays that confirmed non-competitive inhibition: IC₅₀ curves remained unchanged even when ATP concentrations varied tenfold, indicating that NVB does not compete with ATP for binding (2).
Hydrogen-deuterium exchange mass spectrometry (HX-MS) revealed that NVB binds to an allosteric site near the ssDNA-binding channel of the helicase-like domain. This site overlaps the DNA path and blocks the 6–8× ATPase stimulation normally triggered by ssDNA, disrupting Pol θ recruitment and DNA-binding functions in cells (2).
Why it matters: These structural insights are now guiding NVB derivatization and supporting combination therapy strategies with PARP inhibitors for tumors that are HR-deficient or PARPi-resistant.
Beyond WRN and Pol θ: A Broader ATPase Universe
Many ATP-dependent proteins—motor proteins, AAA+ unfoldases, ABC transporters, ubiquitin E1 enzymes, and V-ATPases—are amenable to ADP-Glo-based detection. While kinases have long dominated ATP-focused assays, helicases and other ATPases are quickly gaining attention.
Helicases, in particular, occupy a sweet spot: their ATPase rate closely reflects biological function, they often show tumor-specific dependency and structural data are increasingly available to inform drug development.
Conclusion
From WRN in MSI-H tumors to Pol θ in BRCA-mutant cancers, helicase ATPases illustrate how a simple luminescent readout can illuminate complex therapeutic biology. As more ATP-powered enzymes enter drug discovery pipelines, expect ADP-Glo™ Assays—in both Kinase and Max formats—to keep lighting the way.
Choosing the Right Assay: ADP-Glo vs. ADP-Glo Max
ADP-Glo™ technology provides a versatile platform for quantifying ATPase activity. Depending on the enzyme’s Km for ATP and assay conditions, two formats are available:
| Assay Format | ATP Range | Best For | Cat.# |
| ADP-Glo™ Kinase Assay | ≤1mM | Kinases, non-kinase ATPases with low ATP Km | V6930 |
| ADP-Glo™ Max Assay | ≥1–5 mM | High-turnover ATPases with high ATP Km (e.g., helicases) | V7001 |
Diagram showing ADP-Glo™ Assay protocol:
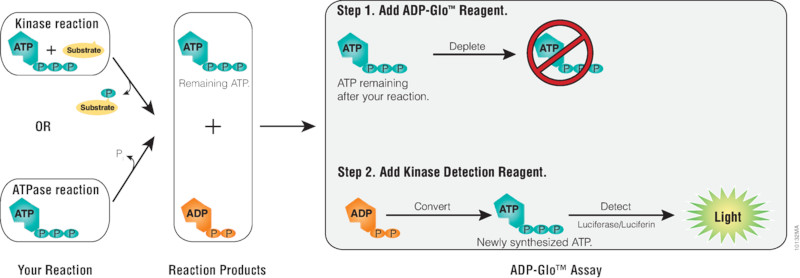
Assay Highlights
- Principle: ADP produced during ATP hydrolysis is converted to ATP, which fuels a luciferase reaction. Luminescence is directly proportional to enzymatic activity.
- Design: Reactions typically include ≤500 nM enzyme, appropriate ATP concentrations, and DNA or protein substrates. Luminescent signal is read in 96- or 384-well plates after 30–120 minutes.
- Hit triage: The signal is mechanism-agnostic—both active-site and allosteric inhibitors appear as loss-of-signal hits. Follow-up assays help identify the mode of inhibition.
- Selectivity: Counter-screens against luciferase inhibitors and unrelated ATPases (e.g., Hsp90) help validate true positives.
| Discover products and technologies to study other targets involved in the DNA Damage Response (DDR) pathway with our Drug Discovery applications page. |
References
1. Moon, B. et al. (2025) Discovery of novel WRN inhibitors for treating MSI-H colorectal cancers. Bioorg. Med. Chem. Lett. 120, 130141.
2. Syed, A. et al. (2023) Novobiocin blocks nucleic acid binding to PolΘ and inhibits stimulation of its ATPase activity. Nucleic Acids Res. 51 (18), 9920-37.
Kari Kenefick
Latest posts by Kari Kenefick (see all)
- ATP-Powered Proteins Beyond Kinases – and Why Helicases Are Stealing the Spotlight - August 7, 2025
- Base Editing Brilliance: David Liu’s Breakthrough Prize and Its Impact - May 28, 2025
- Neurons’ Role in FBP2 Regulation - October 24, 2024
