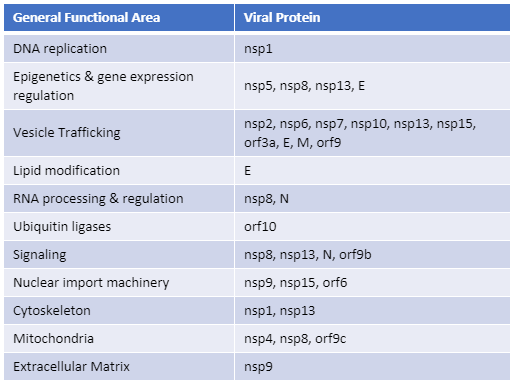
Recently, Gordon et al. published an atlas of protein:protein interactions of all proposed SARS-CoV-2 proteins expressed individually in HEK 293 cells (Table 1). The study tagged each of the viral proteins with an epitope tag and performed a pull-down of the expressed protein followed by trypsin digestion and mass spec analysis, a process referred to as affinity purification–mass spec analysis. The group identified 332 human proteins interacting with 27 SARS-CoV-2 proteins.
The interactions identified in the HEK 293 cells helped Appelberg et al. analyze interactions over time in SARS-CoV-2-infected Huh7 cells. Gordon et al. used the PPI data to identify FDA-approved drugs, drugs in clinical trials, and pre-clinical compounds that bound to the identified human proteins and labs in New York and Paris tested some of these drugs for antiviral effects.