Today’s post is written by Michael Curtin, Senior Product Manager, Reporters and Signaling.
Inflammation is a defense mechanism that the body employs in which the immune system recognizes and removes harmful and foreign stimuli and begins the healing process. Inflammation can be either acute or chronic. Chronic inflammation is also referred to as slow, long-term inflammation and can last for prolonged periods (several months to years); chronic inflammation is caused by immune dysregulation. This typically takes the form of the body’s inability to resolve inflammation resulting from overproduction of inflammatory cytokines and chemokines, as well as danger-associated molecular patterns (DAMPs) released from dying cells (2). Tumor Necrosis Factor (TNF) is the primary cytokine involved in many common inflammatory diseases and is where many therapies targeting inflammation are focused.
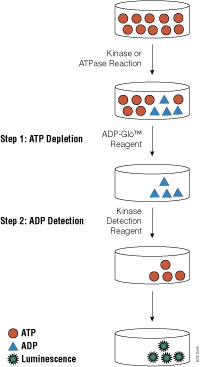
Recent research that RIP kinases (RIPK1 and RIPK3) are important regulators of innate immunity via their key roles in cell death signaling during cellular stress and following exposure to inflammatory and infectious stimuli. RIPK1 has an important scaffolding role in pro-inflammatory signaling where it interacts with TRADD, TRAF1 TRAF2, and TRAF3 and TRADD can act as an adaptor protein to recruit RIPK1 to the TNFR1 complex in a TNF-dependent process. RIPK1 plays a kinase activity-dependent role in both apoptotic and necroptotic cell death. A review article by Speir et al. (1) discusses the role of RIP kinases in chronic inflammation and the potential of RIPK1 inhibitors as a new therapeutic approach for the treatment of chronic inflammation. RIPK1 or Receptor Interacting Protein Kinase 1 is a serine/threonine kinase that was originally identified as interacting with the cytoplasmic domain of FAS. Promega offers several reagents that make studying RIPK1 easier- these include our RIPK1 Kinase Enzyme Systems which includes RIPK1 (Human, recombinant; amino acids 1-327), myelin basic protein (MBP) substrate, reaction buffer, MnCl2, and DTT and is optimized for use with our ADP-Glo Kinase Assay.
Continue reading “RIPK1: Promising Drug Target of Chronic Inflammatory Diseases”![Fig 4. Four point MMOA screen for tideglusib and GW8510. Time dependent inhibition was evaluated by preincubation of TbGSK3β with 60 nM tideglusib and 6 nM GW-8510 with 10μM and 100μM ATP. (A). Tideglusib [60 nM] in 10μM ATP. (B). GW8510 [60 nM] in 10μM ATP. (C.) Tideglusib [60 nM] at 100μM ATP. (D.) GW8510 [60 nM] at 100μM ATP. All reactions preincubated or not preincubated with TbGSK3β for 30 min at room temperature. Experiments run with 10μM GSM peptide, 10μM ATP, and buffer. Minute preincubation (30 min) was preincubated with inhibitor, TbGSK3β, GSM peptide, and buffer. ATP was mixed to initiate reaction. No preincubation contained inhibitor, GSM peptide, ATP, and buffer. The reaction was initiated with TbGSK3β. Reactions were run at room temperature for 5 min and stopped at 80°C. ADP formed was measured by ADP-Glo kit. Values are mean +/- standard error. N = 3 for each experiment and experiments were run in duplicates. Control reactions contained DMSO and background was determined using a zero time incubation and subtracted from all reactions. Black = 30 min preincubation Grey = No preincubation.](https://www.promegaconnections.com/wp-content/uploads/2016/04/journal.pntd_.0004506.g004-243x300.png)