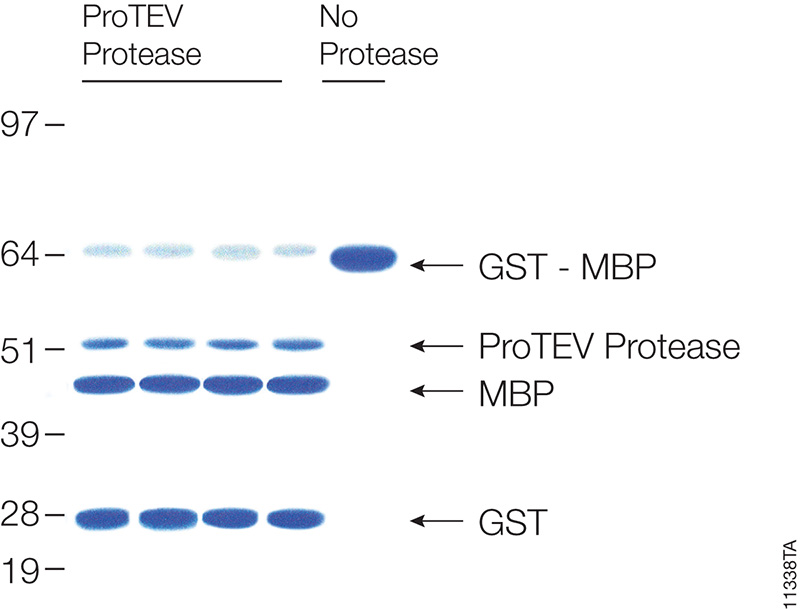
Many proteins are expressed as fusion partners with affinity tags, such as the HaloTag® fusion, glutathione-S-transferase (GST) or maltose binding protein (MBP), to selectively bind the proteins using affinity purification resins. While such resins yield high-purity protein quickly, the large affinity tags are undesirable for some downstream applications. Most expression vectors are designed with a specific protein cleavage site between the two fusion partners to remove the affinity tag after purification. ProTEV Protease recognizes a rare amino acid sequence, EXXYXQ, where X is any amino acid, and cleaves after the glutamine residue.
ProTEV Plus functions over a broad pH and temperature range. In a recent study the enzymatic activity of ProTEV Plus in the presence of various compounds (Table 1) commonly found in protein purification protocols were evaluated.
| Table 1. Test Compounds. | |
| Test Compound |
Final Reaction Concentration |
| Imidazole |
500mM, 50mM, 5mM, 0.5mM |
| Sodium Chloride (NaCl) |
1M, 125mM, 50mM, 10mM |
| Protease Inhibitor Cocktail |
1X, 0.05X |
| Urea |
500mM, 125mM |
| Guanidine-HCl |
500mM, 1mM |
| Glutathione |
2.5mM, 0.01mM |
| Magnesium Chloride (MgCl2) |
100mM, 0.1mM |
| Glycerol |
50%, 1% |
| IGEPAL® CA 630 |
1%, 0.05% |
| Tween™ 20 |
1%, 0.05% |
| Triton™ X-100 |
1%, 0.05% |
| SDS |
5%, 1% |
Table 2 lists the compounds tested and the concentrations at which ProTEV Plus shows ≥94% cleavage. The concentration limits provided are general guidelines because we did not test the compound concentrations in small increments. For some of the compounds analyzed, the highest acceptable concentration may be higher than indicated.
| Table 2. Acceptable Concentrations of ProTEV Plus-Compatible Compounds. | |
| ProTEV Plus-Compatible Compounds |
Acceptable Concentrations* |
| Protease Inhibitor Cocktail |
≤1X* |
| Urea |
≤500mM* |
| Guanidine-HCl |
≤500mM* |
| IGEPAL® CA-630, Tween™ 20, Triton™ X-100 |
≤1%* |
| Glycerol |
≤50%* |
| DTT |
≤10mM* |
| Imidazole |
≤5mM |
| Sodium Chloride |
≤10mM |
| Glutathione |
≤1mM |
| Magnesium Chloride |
≤1mM |
| SDS | All tested concentrations resulted in 0% cleavage. |
| *Indicates the maximum concentration tested for a given compound. | |
For additional details please refer to this article on the Proemga.com Web site.
Are you looking for proteases to use in your research?
Explore our portfolio of proteases today.

Reblogged this on Promega Scientific Training.