Immunometabolism
Welcome to the emerging frontier of immunometabolism. A decade ago, immunology and metabolism were seen as two distinct areas of study. However, we now know that specific metabolic activities are required for proper immune cell differentiation and function. In tumor microenvironments, immune cells may even alter their metabolism to compete with tumor cells for limiting nutrients.
Glucose metabolism in Naïve vs Effector T cells
What does your car and T cells have in common? They both shift gears! You can shift gears on your car to change the way the engine’s power is used to match driving conditions; when you’re going uphill, you switch to a higher gear. Similarly, when T cells are activated, they change the way they generate energy to match functional needs. This makes sense because activated T cells (known as effector T cells) require more energy and biomass to support growth, proliferation and effector functions.
While cars run on gas, the main fuel for T cells is glucose. Each glucose molecule is broken down into pyruvate while generating 2 ATP molecules. Naïve T cells completely oxidize pyruvate through oxidative phosphorylation to generate 36 ATPs per glucose molecule. However, when T cells are activated and become effector T cells, glycolysis is used to produce 2 ATPs per glucose molecule.
Why is there increased glycolysis in effector T cells?
It may seem strange that effector T cells increase the use of glycolysis, which generates less ATP per glucose than oxidative phosphorylation. There may be several reasons for this change. First of all, oxidative phosphorylation is a complex and slow process, requiring multiple steps of electron transport in the mitochondria. Glycolysis, on the other hand, happens in the cytosol and can generate ATP much faster in response to activation. Secondly, glycolysis also produces biosynthetic intermediates that are building blocks for effector T cells undergoing rapid growth and proliferation. These intermediates include glucose-6-phosphate (for nucleotide synthesis), 3-phosphoglycerate (for amino acid synthesis), and pyruvate (for fatty acid synthesis). Furthermore, glycolysis reduces NAD+ to NADH, which is a crucial cofactor for many enzymes that enable cell growth. To sustain the rate of glycolysis, cells often reduce pyruvate to lactate to recycle NADH and maintain NAD+ levels. This causes an increase in lactate levels in effector T cells.
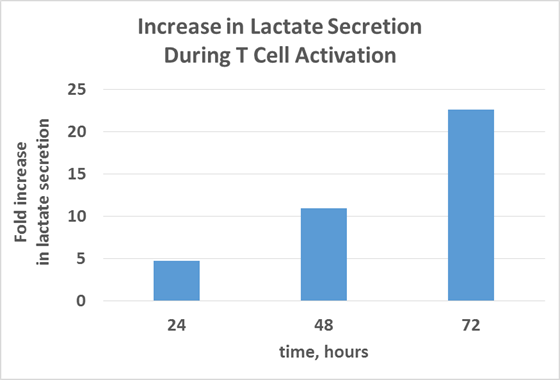
Using Secreted Lactate as a Marker for T cell Activation
This increase in lactate provides a “marker” for monitoring T cell activation. The bioluminescent Lactate-Glo™ Assay enables researchers to monitor the induction of T cell activation easily by looking at the secretion of lactate. In her webinar, “How to Integrate Cellular Metabolism Assays into Your Research: Considerations and Challenges”, Donna Leippe will be describing the Promega portfolio of bioluminescent energy metabolism assays, and specifically illustrating how the Lactate-Glo™ Assay can be used to monitor T Cell activation. You can learn more about the webinar here.
Latest posts by Johanna Lee (see all)
- Can AI Replace High-Throughput Screens for Drug Discovery? - April 30, 2024
- How Avian Influenza Crosses Species - February 22, 2024
- Macrophages: The Unsung Heroes of Immune Response and Biologic Drug Development - December 28, 2023
