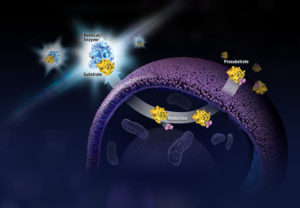
 What if you could uncover a small but significant cellular response as your population of cells move toward apoptosis or necrosis? What if you could view the full picture of cellular changes rather than a single snapshot at one point? You can! There are real-time assays that can look at the kinetics of changes in cell viability, apoptosis, necrosis and cytotoxicity—all in a plate-based format. Seeking more information? Multiplex a real-time assay with endpoint analysis. From molecular profiling to complementary assays (e.g., an endpoint cell viability assay paired with a real-time apoptosis assay), you can discover more information hidden in the same cells during the same experiment.
What if you could uncover a small but significant cellular response as your population of cells move toward apoptosis or necrosis? What if you could view the full picture of cellular changes rather than a single snapshot at one point? You can! There are real-time assays that can look at the kinetics of changes in cell viability, apoptosis, necrosis and cytotoxicity—all in a plate-based format. Seeking more information? Multiplex a real-time assay with endpoint analysis. From molecular profiling to complementary assays (e.g., an endpoint cell viability assay paired with a real-time apoptosis assay), you can discover more information hidden in the same cells during the same experiment.
Whether your research involves screening a panel of compounds or perturbing a regulatory pathway, a more complete picture of cellular changes gives you the benefit of more data points for better decision making. Rather than assessing the results of your experiment using a single time point, such as 48 hours, you could monitor cellular changes at regular intervals. For instance, a nonlytic live-cell reagent can be added to cultured cells and measurements taken repeatedly over time. Pairing a real-time cell health reagent with a detection instrument that can maintain the cells at the correct temperature means you can automate the measurements. These repeated measurements over time reveal the kinetic changes in the cells you are testing, giving a real-time status update of the cellular changes from the beginning to the end of your experiment. Continue reading “Reveal More Biology: How Real-Time Kinetic Cell Health Assays Prove Their Worth”



![Bubonic plague victims in a mass grave in 18th century France. By S. Tzortzis [Public domain], via Wikimedia Commons](https://upload.wikimedia.org/wikipedia/commons/1/1b/Bubonic_plague_victims-mass_grave_in_Martigues%2C_France_1720-1721.jpg)