A widely used molecular biology technique, in vitro transcription uses bacteriophage DNA-dependent RNA polymerases to synthesize template-directed RNA molecules. Enzymes like bacteriophage SP6, T3 and T7 RNA polymerases are used to produce synthetic RNA transcripts, which can be used as hybridization probes, as templates for in vitro translation applications, or in structural studies (X-ray crystallography and NMR). Synthesized RNA transcripts are also used for studying cellular RNA functionality in processes such as splicing, RNA processing, intracellular transport, viral infectivity and translation.
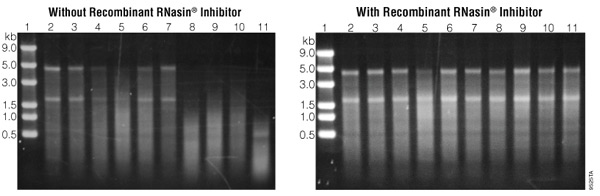
Problems in the transcription reaction can result in complete failure (i.e., no transcript generated) or in transcripts that are the incorrect size (i.e., shorter or longer than expected). Below is a discussion of the most common causes of in vitro transcription problems.
Continue reading “In Vitro Transcription: Common Causes of Reaction Failure”