Depression is not simply a mood disorder, a feeling of sadness, or being ill at ease. Depression can completely shut a person down, manifesting as an inability to make decisions, to take action, to think. Even sleep is affected by depression.
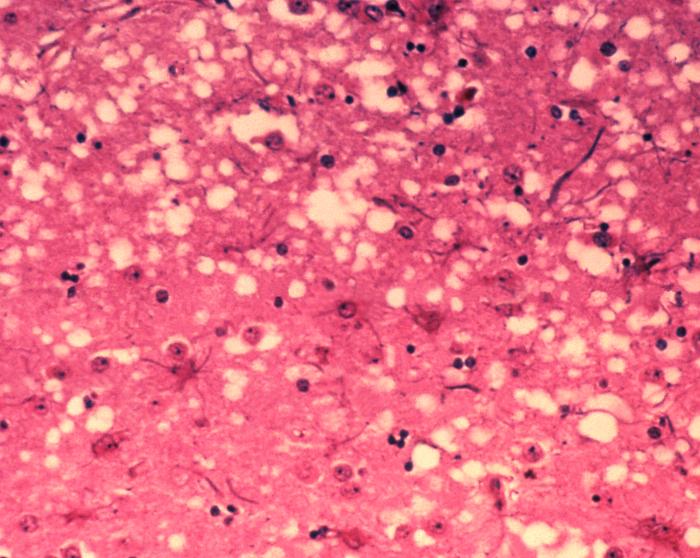
Researchers and clinicians who treat depression are learning that the physical manifestations can be mirrored by internal, cellular changes. Some people with depression have decreases in their gray matter volume, particularly in areas like the hippocampus (important to memory, learning, and emotions) and prefrontal cortex (where higher-level thought and planning abilities are based).
Additionally, imaging has shown a decrease in the number of synapses—the structures through which electrical or chemical signals are passed between neurons and other cells—in persons with chronic depression. Without the signals that synapses transmit, brain function is disrupted.
And without intervention in depression, synapse decrease can continue.
While there are drugs and behavioral therapies to treat depression, these therapies can be slow to act and sometimes ineffective. In addition, once synaptic loss has occurred, these therapies are less effective.
In their August 2021 paper, “Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo” (1), Shao et al. state,
Continue reading “Psilocybin as Antidepressant: Quick Acting, Lasting Benefits”“It has long been recognized that these compounds (serotonergic psychedelics like psilocybin) may have therapeutic potential for neuropsychiatric disorders, including depression, obsessive-compulsive disorder and addiction”.