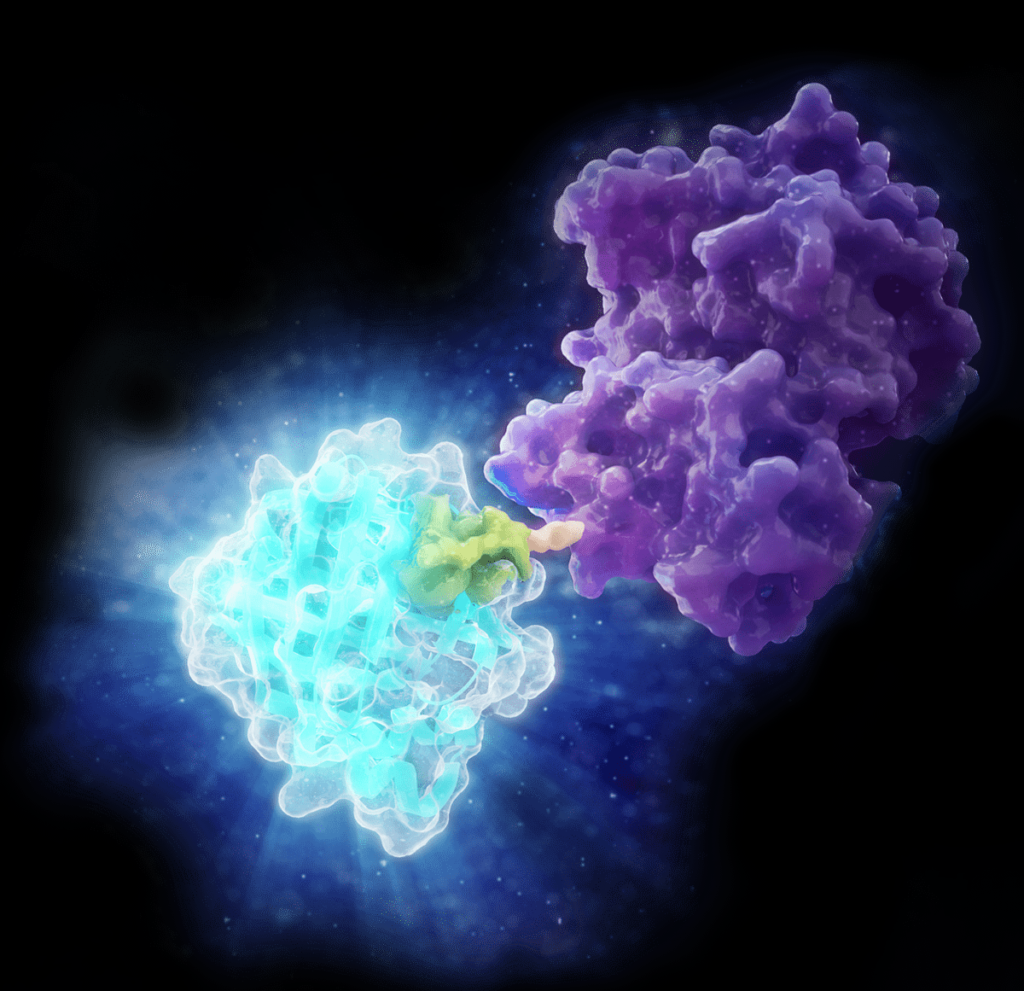
As mRNA therapeutics continue to expand across clinical pipelines, one persistent challenge remains for developers: reducing double-stranded RNA (dsRNA) contaminants that can compromise safety and efficacy. These unintended byproducts of in vitro transcription (IVT) can trigger unwanted immune responses and reduce the potency of the final product. Developers must prioritize dsRNA detection and control as essential steps in the process. In our previous blog post we offered a high-level discussion of what is double-stranded RNA (dsRNA), its biological function, and importance of detection in a therapeutic context. Here, we’ll take a closer look at origins of dsRNA contamination, quality control measures, and improvement strategies.
Large-scale production of single-stranded RNA (ssRNA) for mRNA-based therapeutics is primarily done through in vitro transcription (IVT), an enzymatic process designed to generate high-yield, functional mRNA transcripts from a DNA template. This process uses purified RNA polymerase enzymes, such as T7, that recognize specific promoter sequences in the DNA template, generating the RNA transcripts of interest. However, IVT reactions also generate unwanted dsRNA byproduct. Below, we delve into some of the major quality control (QC) considerations and strategies to reduce dsRNA byproducts.
Continue reading “dsRNA QC Considerations: How I Learned to Stop Worrying and Love my IVT Reactions” Like this:
Like Loading...