Luciferase reporter assays are essential tools in molecular and cellular biology, offering sensitive and quantitative means to study gene expression, transcriptional regulation, signal transduction pathways, and cellular responses to various stimuli. With multiple luciferase reporters and detection reagents available, how do you know which one fits your specific workflow or readout needs?
Choosing a reporter and detection system that aligns with your experimental goals helps you tailor your luciferase reporter assay for the most meaningful results. This blog post will help you navigate the options and key considerations.
Understand Your Luciferase Reporter Options
Luciferases are enzymes that produce bioluminescence through the oxidation of a substrate, emitting light that can be measured using a luminometer. Promega offers three primary luciferase reporters, each with distinct characteristics: Firefly Luciferase, NanoLuc® Luciferase (engineered for deep-sea shrimp luciferase) and Renilla Luciferase. Each luciferase reporter has unique advantages, and the choice depends on factors such as size, brightness and compatibility with extracellular environment. See the table below to compare their key properties:
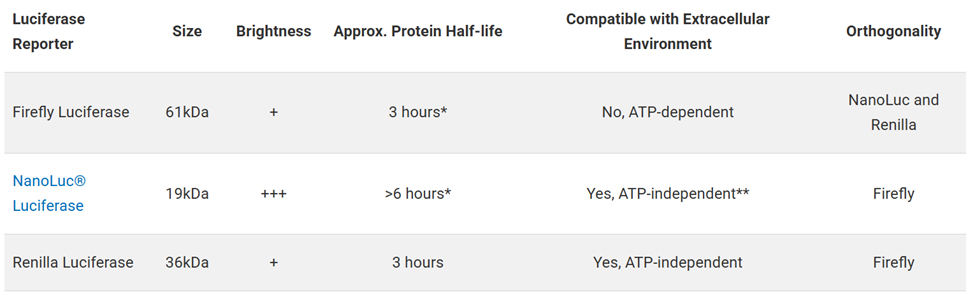
*Destabilized versions available to more tightly couple to transcriptional response.
**Secreted versions available.
Consider the Detection Reagents
Signal stability, or the half-life of the luminescent signal, is another critical consideration, as it can influence your sample processing workflow. Typically, for a given reporter, signal stability and intensity are inversely related, with more progressively decreasing levels of initial brightness observed with respective increases in signal half-life. For example, the Dual-Luciferase® Reporter (DLR®) Assay produces a bright signal with high sensitivity, but the signals that decay quickly—firefly luciferase drops by 50% in about 12–15 minutes, and Renilla luciferase in less than 3 minutes—making it necessary to read samples immediately, often one at a time, or to use luminometers with reagent injectors.
In contrast, the Dual-Glo® Assay provides stabilized luminescence with approximately 2-hour signal half-lives for both reporters, allowing batch processing and compatibility with multiwell plate readers without injectors. However, the signal intensity for both reporters is decreased when compared to the DLR® Assay. By paring firefly luciferase with the bright NanoLuc reporter, the Nano-Glo® Dual-Luciferase® Reporter System (NanoDLR) provides the signal stability observed with the Dual-Glo system, with increased signal intensity for both reporters. This improved design overcomes the brightness tradeoff seen with Dual-Glo® by introducing NanoLuc®, which is over 1000 times brighter than Renilla, and by enhancing firefly luciferase chemistry—resulting in a more robust assay with both stable and high-intensity signals.
Promega offers detection chemistries with a wide range of signal durations—from high sensitivity flash assays like the Luciferase Assay System to glow format systems like Bright-Glo™ System, ONE-Glo™ EX System, Steady-Glo® System, and Nano-Glo® System.
Luciferase Assay Signal Duration Comparison
Here’s how Promega lytic luciferase detection systems compare in signal duration, intensity, and workflow compatibility—so you can quickly identify the one that meets your needs.
| Assay System (Reporter) | Signal Type | Signal Half-Life | Key Characteristics |
| Luciferase Assay System (Fluc) | Flash | ~12–15 minutes | High initial intensity; rapid decay; suitable for quick measurements with injectors. |
| Bright-Glo™ System (Fluc) | Glow | ~30 minutes | Bright signal; moderate stability; ideal for high-throughput applications. |
| ONE-Glo™ System (Fluc) | Glow | ~45 minutes | Robust performance; suitable for high- and ultrahigh-throughput assays. |
| ONE-Glo™ EX System (Fluc) | Glow | ~2 hours | Improved stability; allows for reagent storage and flexible use. |
| Steady-Glo® System (Fluc) | Glow | ~5 hours | Long-lasting signal; ideal for batch processing in multiwell plates. |
| Nano-Glo® System (Nluc) | Glow | >2 hours | Ultra-bright and stable; suitable for low to high-throughput processing. |
| Dual-Luciferase® (DLR®) System (Fluc/Rluc) | Flash | Fluc: ~12–15 minRluc: ~2–3 min | Requires immediate reading; suitable for small sample numbers with maximum sensitivity. |
| Dual-Glo® System (Fluc/Rluc) | Glow | ~2 hours (both reporters) | Stabilized signals; compatible with multiwell plate readers without injectors. |
| Nano-Glo® Dual-Luciferase® (NanoDLR) System (Fluc/Nluc) | Glow | ~2 hours (both reporters) | Stabilized signals; high sensitivity for both reporters. |
* Fluc refers to firefly luciferase; Rluc refers to Renilla luciferase.
It’s also important to evaluate the complexity of the assay protocol. Non-homogeneous assays involve an additional step to create a lysate before adding the detection reagent, which can extend assay time and introduce variability. In contrast, homogeneous assays simplify the workflow by allowing direct addition of the reagent to cells in culture, eliminating the need for sample pre-processing.
Lastly, consider whether you need a lytic or live-cell reporter detection system. While this blog post focuses on lytic assays, live-cell luciferase formats for NanoLuc® and Renilla reporters are also available to support real-time and longitudinal analyses. Consider also whether your experiment requires single-reporter or dual-reporter detection, the latter of which can be used for assay normalization or to measure two distinct biological responses in parallel. Tailoring your selection based on these factors will help ensure a luciferase assay that aligns closely with your experimental goals.
To explore and compare luciferase assay characteristics, and find the best fit for your next experiment, visit our Luciferase Assay Selection Guide.
Riley Bell
Latest posts by Riley Bell (see all)
- Bones and Blood: Uncovering History Through DNA - October 31, 2025
- Matching Luciferase Reporter Assays to Your Experimental Goals - August 5, 2025
- How to Choose a Luciferase Reporter Assay - May 23, 2025
