As mRNA therapeutics continue to expand across clinical pipelines, one persistent challenge remains for developers: reducing double-stranded RNA (dsRNA) contaminants that can compromise safety and efficacy. These unintended byproducts of in vitro transcription (IVT) can trigger unwanted immune responses and reduce the potency of the final product. Developers must prioritize dsRNA detection and control as essential steps in the process. In our previous blog post we offered a high-level discussion of what is double-stranded RNA (dsRNA), its biological function, and importance of detection in a therapeutic context. Here, we’ll take a closer look at origins of dsRNA contamination, quality control measures, and improvement strategies.
Large-scale production of single-stranded RNA (ssRNA) for mRNA-based therapeutics is primarily done through in vitro transcription (IVT), an enzymatic process designed to generate high-yield, functional mRNA transcripts from a DNA template. This process uses purified RNA polymerase enzymes, such as T7, that recognize specific promoter sequences in the DNA template, generating the RNA transcripts of interest. However, IVT reactions also generate unwanted dsRNA byproduct. Below, we delve into some of the major quality control (QC) considerations and strategies to reduce dsRNA byproducts.
QC Challenge # 1: Template and Sequence Design
Pitfall:
A significant starting point for preventing dsRNA is careful template and sequence design. When the DNA template contains repetitive or highly complementary regions, partial self-annealing can occur during transcription, creating zones where dsRNA can form. GC-rich stretches and secondary structures also invite polymerase stalling and truncated transcripts, which can align and form double-stranded segments1.
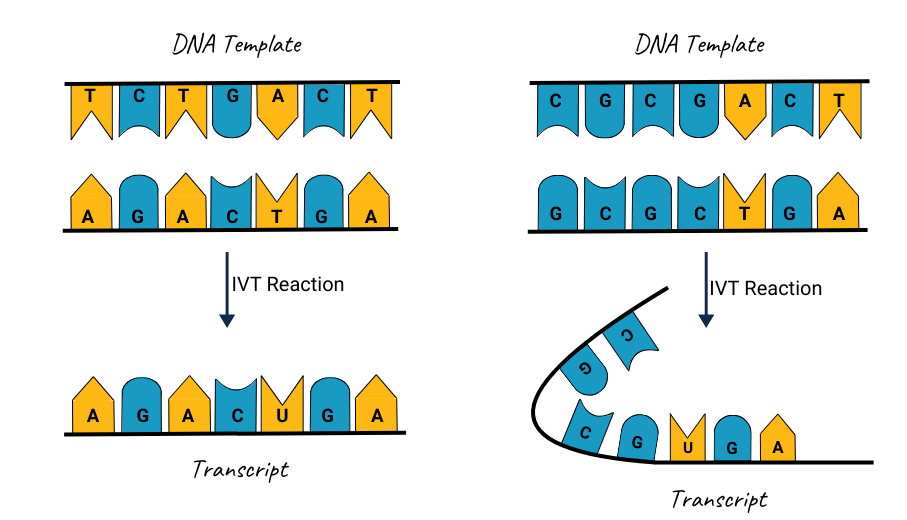
Improvement:
To counter these problems, codon optimization and the removal of repetitive elements can help ensure a smoother transcription process. It is also important to confirm that the plasmid is fully linearized at the correct cut site. Even minimal amounts of nicked or supercoiled DNA can undermine the transcription reaction, leading to inconsistent results and a higher risk of dsRNA formation.
QC Challenge # 2: Reaction Conditions
Pitfall:
Optimal reaction conditions are another key factor. If the IVT is performed at an incorrect temperature or duration, there is a greater likelihood of incomplete or aberrant transcripts that may form dsRNA. Similarly, concentrations of reaction components, like salts, magnesium, and nucleotides, must be closely monitored to ensure that the polymerase operates at peak efficiency without introducing errors2.
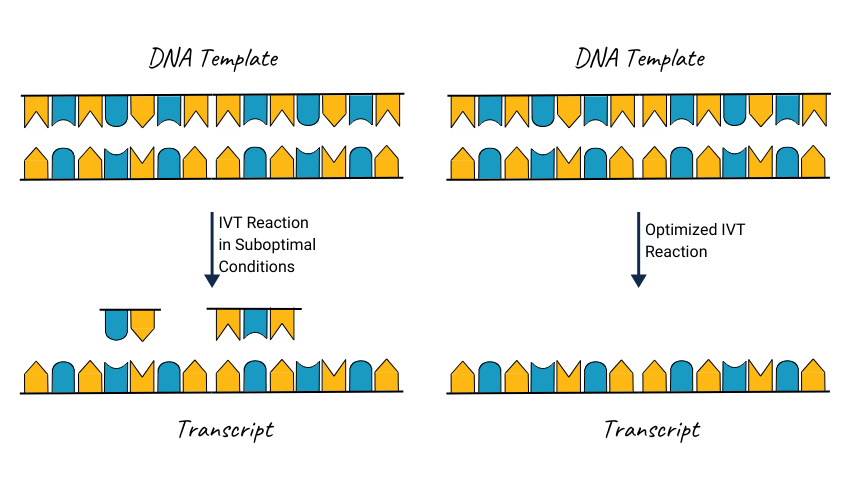
Improvement
Polymerase fidelity plays a crucial role in the overall product quality. Using high-fidelity enzymes, quality reaction mixtures, proper Mg²⁺ levels for optimized engineered T7 polymerase function, the IVT reaction progresses much more efficiently without generating aberrant products. During the research phase of development, it is important to consider the sourcing of critical reagents for downstream development, like the cGMP mRNA materials offered by Promega, as uncontaminated reagents prevent unintended reaction conditions that can result in transcripts prone to annealing and dsRNA formation. Identifying those critical reagents early on can be beneficial during the transition to later stages.
QC Challenge #3: Functional Activity and Potency
Pitfall:
Even with optimization and mitigation strategies around the starting template and reaction conditions, there is no guarantee that the RNA product is free from dsRNA contamination. A thorough QC detection strategy for post-IVT dsRNA is essential, if it does slip through these safeguards. Reliance on a single readout can easily overlook dsRNA byproducts. Common methods such as enzyme-linked immunosorbent assays (ELISAs) and dot blots using dsRNA-specific monoclonal antibodies (such as J2 or K1) have serious drawbacks as their performance is heavily dependent on the antibody binding attributes, which may not accurately quantify dsRNA species3.
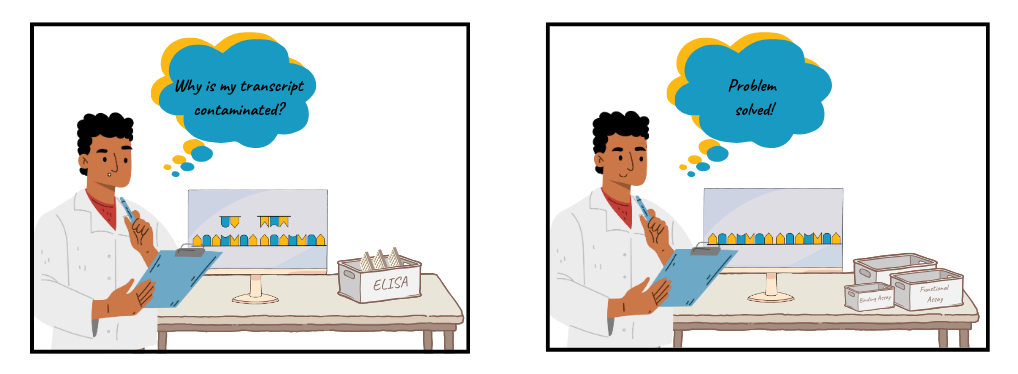
Improvement:
Instead, scientists should employ more sensitive methods to verify the purity of IVT products: a combination of antibody-independent binding assays and functional assays is recommended. Biochemical binding assays, such as the Lumit dsRNA Detection Assay and the cell-based TLR3 Bioassay, offer critical functional insights into the purity of the IVT product through accurate, sensitive quantitative and biologically relevant innate immune activation, respectively.
Continiue Exploring dsRNA Challenges and Solutions
Reducing dsRNA contamination requires careful attention across every stage of the IVT workflow, from template design and reaction setup to final quality control. In this post, we highlighted common sources of dsRNA byproducts and outlined strategies to improve RNA quality for downstream applications. This blog is part of our ongoing series on double-stranded RNA in mRNA therapeutics. If you missed our first post, be sure to check out our discussion on the biological role of dsRNA and why detection is critical. Stay tuned for the final blog in the series, where we’ll examine how chemically modified nucleotides support therapeutic performance—and introduce new challenges for dsRNA detection and quantification.
References
- Arnaud-Barbe, N., V. Cheynet-Sauvion, G. Oriol, B. Mandrand, & F. Mallet. (1998) Transcription of RNA templates by T7 RNA polymerase. Nucleic Acids Res. 26: 3550-3554. ↩︎
- Guo, L., Z. Liu, S. Song, W. Yao, M. Yang, & G. Chen. (2024) Maximizing the mRNA productivity for in vitro transcription by optimization of fed-batch strategy. Biochem Engineering Journal. 210: 109412. ↩︎
- Bonin, M., J. Oberstrass, N. Lukacs, K. Ewert, E. Oesterschulze, R. Kassing, & W. Nellen. (2000) Determination of preferential binding sites for anti-dsRNA antibodies on double-stranded RNA by scanning force microscopy. RNA. 6: 563-570. ↩︎
Anna Bennett
Latest posts by Anna Bennett (see all)
- A New View of Protein Degradation with HiBiT and Live Cell Imaging - September 23, 2025
- HiBiT-Based NanoBRET® Assay Sheds Light on GPCR–Ligand Binding in Live Cells - July 31, 2025
- Understanding Wnt Signaling Through β-Catenin Localization in Live Cells - June 19, 2025
