Glycosylation is the process by which a carbohydrate is covalently attached to target macromolecules, typically proteins. This modification serves various functions including guiding protein folding (1,2), promoting protein stability (2), and participating signaling functions (3).
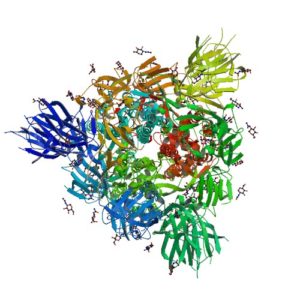
SARS-CoV-2 utilizes an extensively glycosylated spike (S) protein that protrudes from the viral surface to bind to angiotensin-converting enzyme 2 (ACE2) to mediate host-cell entry. Vaccine development has been focused on this protein, which is the focus of the humoral immune response. Understanding the glycan structure of the SARS-CoV-2 virus spike (S) protein will be critical in the development of glycoprotine-based vaccine candidates.
In a recent publication from Wantanabe and colleagues, the site-specific glycosylation of SARS-CoV-2 and the glycan compositions were determined for all 22 N-linked glycan sites located on the S protein (4) using mass spectrometry.
The basic experimental design included the following steps:
- Expression of the SARS-CoV-2 S protein in human embryonic kidney 293F cells
- Purification of recombinant protein,
- Sample prep and digestion with trypsin or chymotrypsin (Promega), and
- Analysis by Liquid chromatography-mass spectrometry (LC/MS).
This glycosylation analysis of SARS-CoV-2 offers insight into the site-specific glycan content of the S protein. Glycoprotein-based vaccines for SARS-CoV-2 are a growing area of focus, and this information can aid in understanding immunogen integrity of vaccine candidates as they are developed.
Try a sample of high-efficiency Trypsin Platinum today and visit our website for more on Trypsin, Mass Spectrometry Grade, with enhanced proteolytic efficiency and superior autoproteolytic resistance.
Interested in tools and resources for viral research? Visit our web page on SARS-CoV-2 Research, Vaccine, and Therapeutic Development.
Literature Cited
- Xu, C. and Ng, D.T.W. (2015) Glycosylation-directed quality control of protein folding. Nat. Rev. Mol. Cell Biol. 16, 742–52.
- Jayaparakash, N. and Surolia, A. (2017) Role of glycosylation in nucleating protein folding and stability. Biochem J. 474, 2333–47.
- Dewald, J.H. et al. (2016) Role of cytokine-induced glycosylation changes in regulating cell interactions and cell signaling in inflammatory diseases and cancer. Cells, 5. 10.3390/cells5040043
- Watanabe, Y. et al. (2020) Site Specific glycan analysis of the SARS-CoV-2 spike Science May 20.
