Researchers having been sharing plasmids ever since there were plasmids to share. Back when I was in the lab, if you read a paper and saw an interesting construct you wished to use, you could either make it yourself or you could “clone by phone”. One of my professors was excellent at phone cloning with labs around the world and had specific strategies and tactics for getting the plasmids he wanted. Addgene makes this so much easier to share your constructs from lab to lab. Promega supports the Addgene mission statement: Accelerate research and discovery by improving access to useful research materials and information. Many of our technology platforms like HaloTag® Fusion Protein, codon-optimized Firefly luciferase genes (e.g., luc2), and NanoLuc® Luciferase are present in the repository. We encourage people to go to Addgene to get new innovative tools. Afterall, isn’t science better when we share?
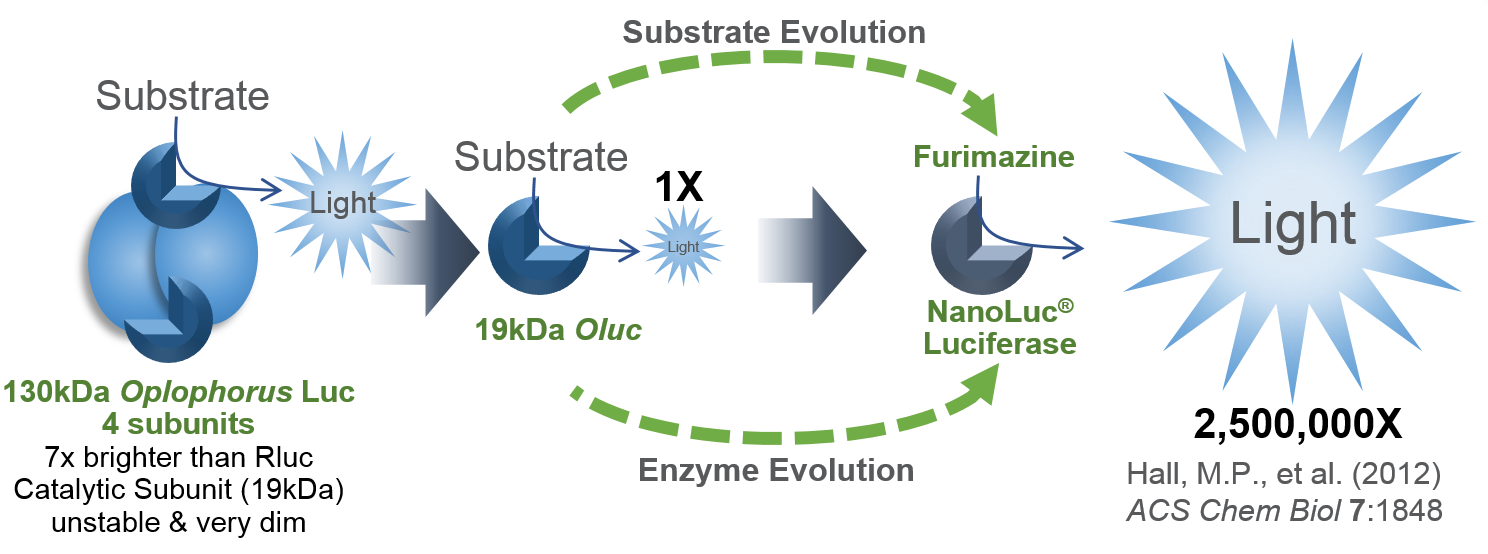
I’d like to focus on some tools in the Addgene collection based on NanoLuc® Luciferase (NLuc). The creation of NanoLuc® Luciferase and the optimal substrate furimazine is a good story (1). From a deep sea shrimp to a compact powerhouse of bioluminescence, NLuc is 100-fold brighter than our more common luciferases like firefly (FLuc) and Renilla (RLuc) luciferase. This is important not so much for how bright you can make a reaction but for how sensitive you can make a reaction. NLuc requires 100-fold less protein to produce the same amount of light from a Fluc or RLuc reaction. NLuc lets you work at physiological concentrations. NLuc is bright enough to detect endogenous tagged genes generated through the CRISPR/Cas9 knock-in. NLuc is very inviting for endogenous tagging as it is only 19kDa. An example is the CRISPaint-NLuc construct (Plasmid #67178) for use in the system outlined in Schmid-Burgk, J.L. et al (2).
Two applications of NanoLuc® Technology have caught my attention through coupling the luciferase with fluorescent proteins to make better imaging reporters and biosensors.
NanoLuc®-Fluorescent Protein Fusions for Imaging
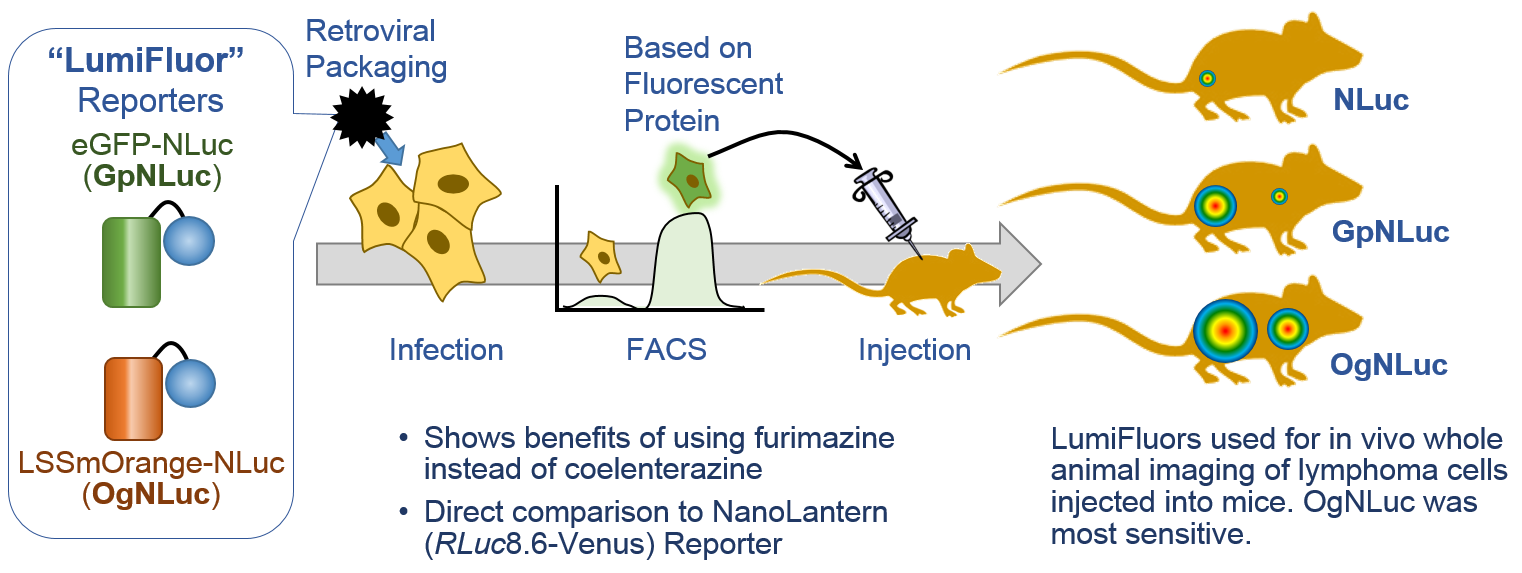
The brightness of NLuc has caught the attention of researchers wanting to perform whole-animal imaging, however, the light is at a maximum around 460nm. For good imaging, the light should be near 600nm to avoid absorbance by heme-containing proteins. Fluorescent protein use in vivo is limited because the proteins must be excited by an external light source, which generates autofluorescence and has limited penetration due to absorption by tissues. Bioluminescence imaging continues to be a solution, especially Fluc (612nm emission at 37°C), but its use typically requires long image acquisition times. These labs use NLuc to excite a red-shifted fluor to get the low background of bioluminescence with the high tissue penetration of light above 600nm. This is bioluminescent resonance energy transfer or BRET.
LumiFluors. Schaub, F.X. et al. (3) designed a imaging probe fusing NLuc with an enhanced green fluorescent protein (GpNluc) and a more penetrative orange fluorescent protein fusion with NLuc (OgNLuc). The lab has dubbed these LumiFluor Reporters and both GpNluc (Plasmid #70185) and OgNluc (Plasmid #70186) are available.
Antares. Chu, J. et al. (4), likewise, wanted to produce a BRET probe for imaging. They started by designing a cyan-excitable orange fluorescent protein (CyOFP1) from mNeptune. Next, NLuc was sandwiched between two copies of CyOFP1. The extremely bright BRET protein was named Antares (Plasmid #742799) after the binary star system.
Enhanced Nano-Lanterns. Though not for deep tissue imaging, NLuc-based BRET reporters created a rainbow of choices for cellular imaging. Suzuki, K. et al. (5) produced multiple color variants for real-time imaging of cellular processes. The Turquoise (CeNL; Plasmid #85199), Yellow (YeNL; Plasmid #85201), Orange (OeNL; plasmid #85202) and Red (ReNL; plasmid #85203) NLuc fusions are available as well as more than 25 fusions to other cellular proteins.
BRET-Based Biosensors Using NanoLuc® Luciferase
Many intracellular sensors of events like calcium release have been made using fluorescent resonance energy transfer (FRET). Basically, you have responsive protein like a calcium binding protein with two fluorescent proteins attached. Binding of calcium by the responsive protein changes conformation and brings the two protein together and improves the FRET signal. The sensors are measured by hitting the most blue-shifted fluorescent protein with its excitation wavelength (donor). The resulting emission is transferred to the most red-shifted fluorescent protein in the pair, and the result is ultimately emission from the red-shifted protein (acceptor).
FRET sensors face challenges of photobleaching, autofluorescence, and, in the case of exciting cyan-excitable donors, phototoxicity. Another challenge to using FRET sensors comes when employing optogenetic regulators to initiate the event you wish to monitor. Optogenetic regulators respond to specific wavelengths and initiate signaling. The challenge comes when the FRET donor excitation overlaps with the optogenetic initiation wavelengths. Researchers have sought to alleviate many of these challenges by exchanging the fluorescent donor for a bioluminescent donor, making bioluminescence resonance energy transfer (BRET) probes. NLuc is an excellent BRET donor due to the bright luminescence.
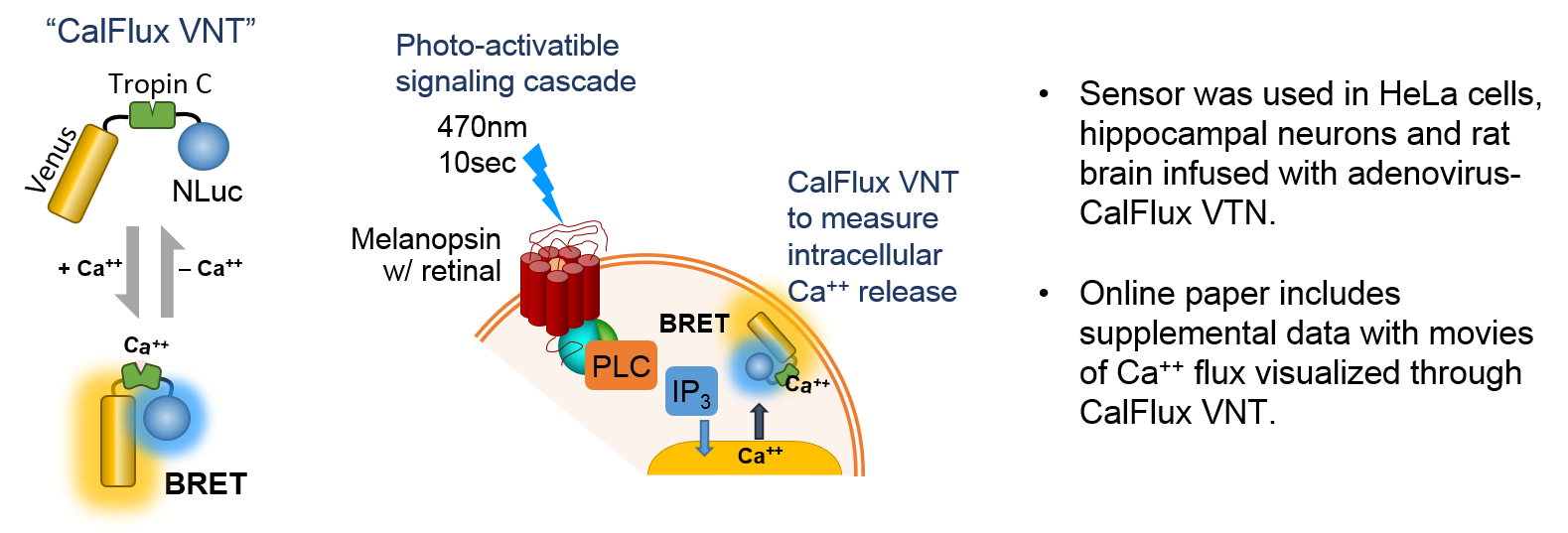
Intracellular Calcium Sensor: Yang, J. et al. (6) wished to trigger intracellular Ca2+ release with a optogenetic receptor, melanopsin, requiring brief exposure to 470nm blue light. A probe was needed that had some very specific characteristics as outlined by the authors: did not require photoexcitation, was ion-specific, sensitive, expressible and gave ratiometric data. The result was a molecule called CalFlux VNT. CalFlux VNT is composed of Venus fluorescent protein, Troponin C Ca2+ binding domain, and NanoLuc® Luciferase. The CalFlux VNT responded to changes in intracellular calcium and was useful for direct imaging of the events in cells and brain explants. This lab (Carl H. Johnson, Vanderbilt University) invented the BRET technique. This construct is available through Addgene (Plasmid #83926).
Live-Cell Voltage Sensor: Ingaki and colleagues (7) were interested in using photoactivatable, optogenetic accuators to depolarize or hyperpolarize membranes. Traditional fluorescent FRET reporters could not be used as the excitation wavelengths needed would also activate the optogenetic accuators. The researchers took a fluorescent FRET reporter and adapted it into a BRET reporter by using NanoLuc® Luciferase to excite the Venus fluorescent protein in conjunction with a membrane-inserted voltage sensing domain (LOTUS V; Luminescent Optical Tool for Universal Sensing of Voltage). Depolarized membranes allow the BRET reaction and hyperpolarized membranes separate the two and no BRET occurs. The authors demonstrate how LOTUS V works in many model systems. This construct is available through Addgene (Plasmid # 87127).
Pairing NanoLuc® luciferase with fluorescent proteins has allowed researchers to make innovative tools not possible with other luciferases. The bright blue luminescence of NanoLuc luciferase has been shifted to more imaging-friendly wavelengths for cellular and animal imaging through fusion with fluorescent proteins. FRET sensors were converted to BRET sensors to avoid adverse side-effects and allow use of optogenetic regulators. Fortunately, these labs have seen fit to share their tools with others through Addgene.
Literature Cited
- Hall, M.P. et al. (2012) Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate. ACS Chem Biol 7, 1848–57.
- Schmid, J.L. et al. (2016) CRISPaint allows modular base-specific gene tagging using a ligase-4-dependent mechanism. Nature Comm. 7, 12338.
- Schaub, F.X., et al. (2015) Fluorophore-NanoLuc BRET reporters enable sensitive in vivo optical imaging and flow cytometry for monitoring tumorigenesis. Cancer Res. 75, 5023–33.
- Chu, J. et al. (2016) A bright cyan-excitable orange fluorescent protein facilitates dual-emission microscopy and enhances bioluminescence imaging in vivo. Nature Biotechnol. 34, 760–7.
- Susuki, K. et al. (2016) Five colour variants of bright luminescent protein for real-time multicolour bioimaging. Nature Comm. 7, 13718.
- Yang, J. et al. (2016) Coupling optogenetic stimulation with NanoLuc-based luminescence (BRET) Ca++ sensing. Nature Comm. 7, 13268.
- Inagaki, S. et al. (2017) Genetically encoded bioluminescent voltage indicator for multi-purpose use in wide range of bioimaging. Reports 7, 42398.