Peptide Predicament
For decades now, peptides have been a molecule of interest for drug discovery research. Peptides offer a unique opportunity for therapeutic intervention that closely mimics natural pathways, as many physiological functions utilize peptides as intrinsic signaling molecules. Macrocyclic peptides, in particular, have recently proven to be promising candidates for targeting intracellular protein–protein interactions (PPIs), an attractive but hard-to-reach therapeutic target for conventional small molecule and biological drugs.
As with any opportunity, there are also challenges that accompany the peptide therapeutic development. Peptide ligands typically have poor membrane permeability, so thus far the majority of peptide therapeutics predominantly target extracellular proteins and receptors. There are also multiple mechanisms for cellular uptake of peptides, including both energy-dependent routes like endocytosis, and energy-independent, like passive diffusion or membrane translocation. Multiple mechanisms of cellular uptake paired with poor permeability makes engineering enough membrane permeability into peptides in order to advance them through drug discovery pipelines extremely difficult.
There are other factors to consider in developing peptide therapeutics, such as solubility, protein/lipid binding and stability, which can also have an affect on the overall cytosolic concentration and, ultimately impact the ability of the peptide to effectively engage its desired intracellular targets.
With so many challenging factors, the ability to have a predictive, high-throughput assay to assess cell permeability, independent of the mechanism(s) of entry, would be a critical and invaluable tool to support peptide drug discovery research.
In a recent study published in ACS Chemical Biology, researchers sought to develop such a tool, and demonstrated a new application for Promega NanoBRET™ technology: the NanoClick assay.
Permeability Possibilities
The NanoClick assay is a target-agnostic cell permeability assay that measures the relative cumulative cytosolic exposure of a peptide in a concentration-dependent manner. The name NanoClick is derived from a combination of its main components: Click chemistry and an intracellular NanoBRET™ signal.
The NanoClick assay is built on three underlying technologies—in-cell copper-free Click chemistry and Promega’s HaloTag® and NanoBRET™ technologies. Specifically, the assay monitors permeability of azide-labeled peptides in cells expressing Promega’s NanoLuc-HaloTag protein. It uses a multi-well plate format that’s compatible with high-throughput applications.
The first step in a NanoClick assay is the cellular expression of the NanoLuc-HaloTag fusion protein. HaloTag is a modified bacterial haloalkane dehalogenase, and reacts with chloroalkane-containing (CA) ligands to form a covalent HaloTag-ligand conjugate—a reaction that is fast and irreversible under physiological conditions. The HaloTag® technology has been used extensively to measure protein-protein interaction in cells and was adapted to the NanoClick assay.
The next step involve incubating the cells expressing the NanoLuc-HaloTag protein with DIBAC-Chloroalkane (CA) molecules. The CA will covalently react with the HaloTag® portion of the fusion protein, thereby labeling the NanoLuc-HaloTag protein with DIBAC. It’s this labeling with DIBAC that allows subsequent ligation of Azide-containing molecules via in-cell copper-free Click chemistry.
The in-cell Click reaction chemistry offers multiple advantages over existing cell-based methods, like bioorthogonality (very narrow and specific reactivities), biocompatibility (doesn’t require cytotoxic Cu(I) catalysts), and utilization of chemically/thermally stable reagents. The reaction also results in quantitative yields of stable triazoles and the reactive azide handle is small and has presumably minimal effect on the peptide physiochemical properties, unlike many cell-based approaches which require conjugation of a large, hydrophobic fluorescent tag to monitor cellular uptake of the peptide of interest which can alter its molecular weight and physiochemical properties. The BRET acceptor, NanoBRET™ 618-Azide dye, is subsequently added in excess and reacts with remaining DIBAC-labeled NanoLuc-HaloTag proteins.
The last step involves the addition of Intracellular TE Nano-Glo® Substrate/Inhibitor and detection of the BRET signal. The NanoLuc® substrate is used by NanoLuc® luciferase to produce light that can be absorbed by the NanoBRET™ 618-Azide dye, resulting in a BRET signal. The extracellular NanoLuc® inhibitor that is added at the same time as the substrate, ensures that the signal arises from inside live cells. In this assay, the absence of a peptide produces a high BRET signal and the presence of a cell-permeable peptide results in a low BRET signal.
NanoClick Assay Overview
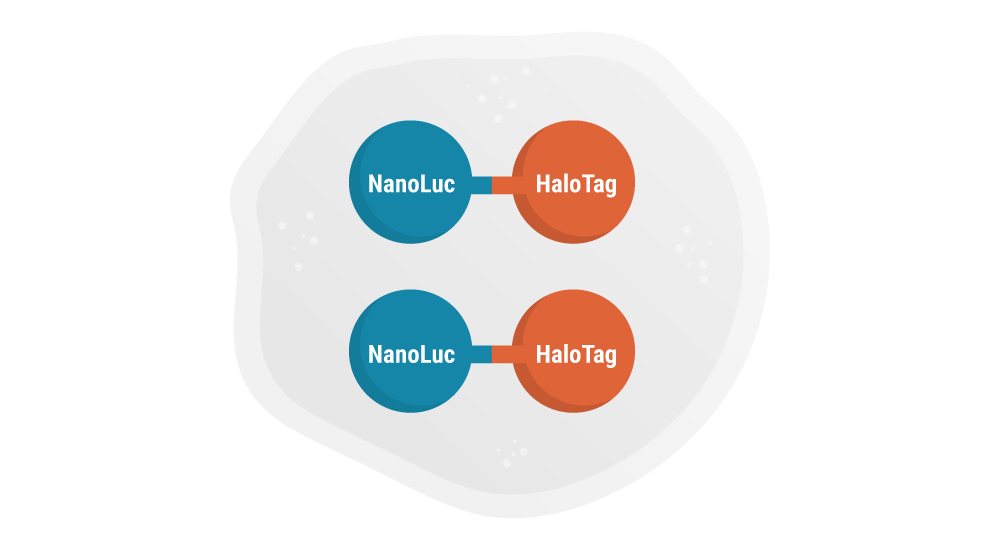
Express NanoLuc-HaloTag Fusion Protein in Cells
Cells are transfected with a DNA construct that encodes for expression of the NanoLuc-HaloTag fusion protein in the cytoplasm.
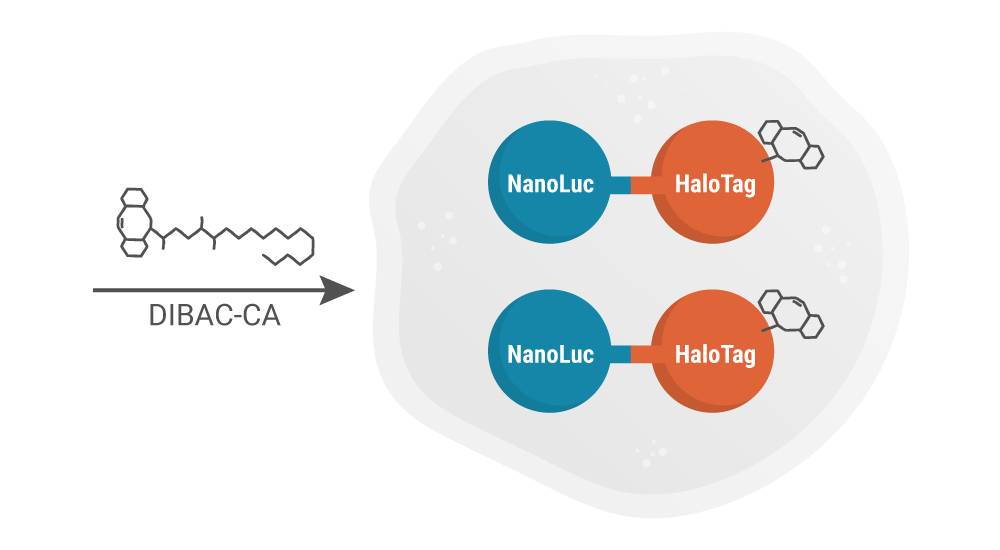
Add DIBAC-CA to Cells
Cells are incubated with dibenzoazacyclooctyne-chloroalkane (DIBAC-CA), during which the HaloTag covalently bonds with the CA linker.
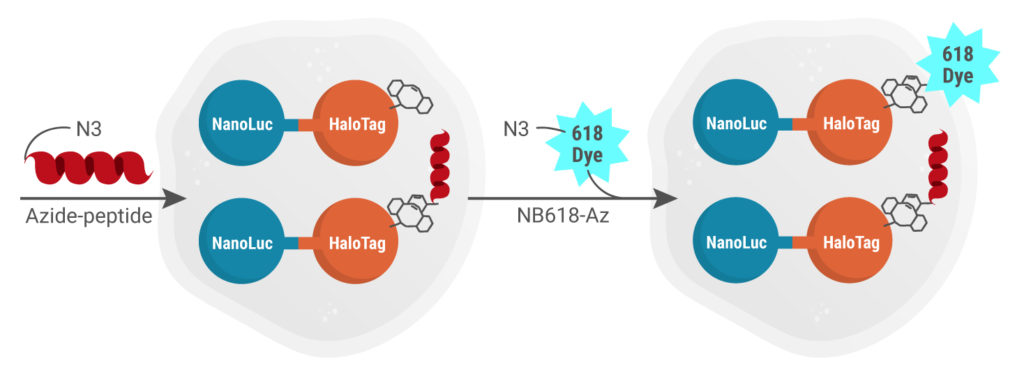
Copper-Free In-Cell Click Chemistry
Azide-labeled peptide dissolved in assay buffer in added. If the peptide is permeable, it will covalently bind the DIBAC-labeled HaloTag. Then excess NanoBRET™ 618-Azide dye is added, which will react with any available unoccupied DIBAC molecules linked to the HaloTag site via the CA moiety in cells.
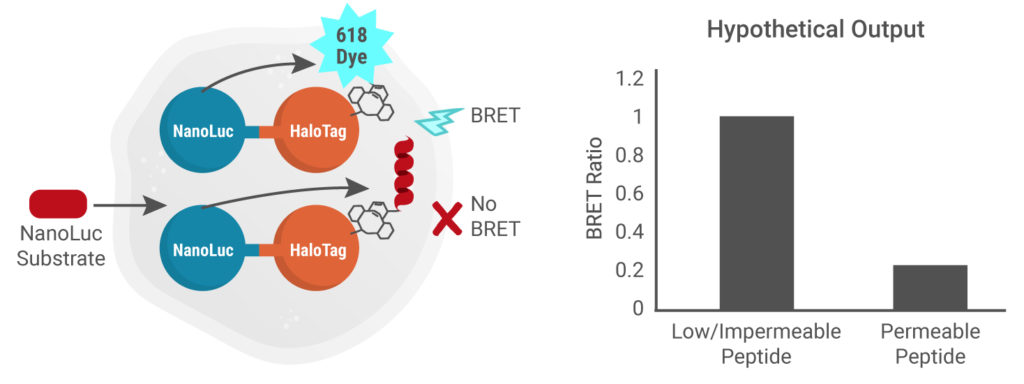
Add Intracellular TE Nano-Glo® Substrate/Inhibitor
NanoLuc substrate is added to cells, allowing measurement of the intracellular NanoBRET™ signal. Impermeable peptide = High NanoBRET™ signal
Presence of cell-permeable peptide = Low NanoBRET™ signal
NanoClick Assay: Promising Predictive Potential
By measuring the ability of peptides to enter cells via different mechanisms, such as endocytosis, passive permeabilty or membrane translocation, the NanoClick approach offers a valuable—and adjustable—new method for drug discovery. Though this study reports the specific application of measuring cellular peptide permeability, the assay can be easily adapted to measure uptake of other molecular targets, such as macromolecules, nucleic acids, proteins and nanotherapeutics. The NanoClick approach can be employed as a screening tool, assisting with uncovering predictive design rules that can then inform and guide structure-activity-permeability relationships for optimization of functionally active molecules.
Check out the full methods for the NanoClick assay with NanoBRET™ technology in the recent publication in ACS Chemical Biology.
Peier, A. et al. (2021) NanoClick: A High Throughput, Target-Agnostic Peptide Cell Permeability Assay. ACS Chem. Bio. 16, 293—309.
NanoBRET™ Technology has a wide range of applications beyond studying protein-protein interactions and drug discovery. Learn more in these blog posts:
- NanoBRET™ Assays to Analyze Virus:Host Protein:Protein Interactions in Detail
- CRISPR/Cas9, NanoBRET and GPCRs: A Bright Future for Drug Discovery
- About the Development of an Improved BRET Assay: NanoBRET

Is the plasmid / lenti vector encoding the NanoLuc-Halo Tag construct available to purchase?
The construct used in the NanoClick assay was the NanoBRET™ Positive Control vector, Cat.# N1581. https://www.promega.com/products/protein-interactions/live-cell-protein-interactions/nanobret-positive-control/
Cat#’s for other reagents key to the NanoClick assay if they ask, e.g.
• NanoBRET 618 dye
https://www.promega.com/c/global/forms/diy-nanobret-target-engagement-assay-materials/ The assay is labeling HaloTag with DIBAC-CA. Azide-labeled peptides will bind to the DIBAC-CA. The sites without peptide binding will be labeled with NanoBRET-Azide dye to measure the amount of binding
• Intracellular TE Nano-Glo® Substrate/Inhibitor, Cat.# N2162 https://www.promega.com/products/cell-signaling/kinase-target-engagement/intracellular-te-nano-glo-substrate-inhibitor/
If you have additional questions please reach out.