With the COVID-19 pandemic far from over in the United States and worldwide, the battle against the disease continues to intensify. Much hope has been pinned on vaccine development. However, vaccines are a long-term, preventative strategy. The immediate need for drugs to fight COVID-19 has accelerated efforts for a variety of potential treatments (see The Race to Develop New Therapeutics Against Coronaviruses).
The Remdesivir Origin Story
One drug that has received widespread attention is remdesivir. It was developed from research by Gilead Sciences that began in 2009, originally targeting hepatitis C virus (HCV) and respiratory syncytial virus (RSV) (1). At present, remdesivir is classified as an investigational new drug (IND) and has not been approved for therapeutic use anywhere in the world.
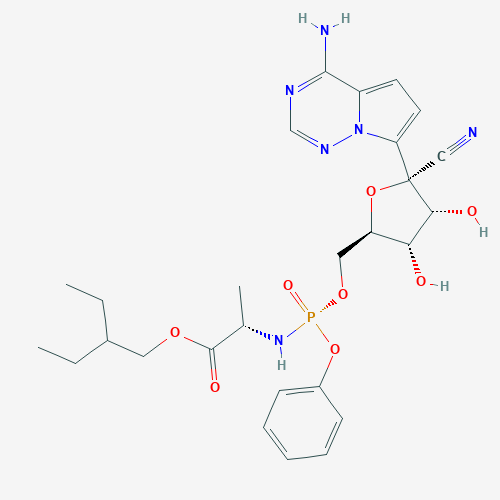
Remdesivir is a prodrug of an adenosine triphosphate (ATP) analog; i.e., when metabolized, it is converted to an active form that mimics the structure of an ATP molecule. Nucleoside analogs have been used to treat a variety of diseases caused by retroviruses like HCV and human immunodeficiency virus (HIV), as well as some coronaviruses—the virus family to which SARS-CoV-2 belongs.
Because remdesivir resembles ATP, it can compete with ATP for incorporation into the growing strand of RNA during viral replication. However, since it is not an ATP molecule, it inhibits the viral RNA-dependent RNA polymerase (RdRp) upon binding, thereby shutting down RNA replication (2). Therefore, remdesivir can reduce the number of virus particles produced in an infected cell.
Remdesivir was initially tested in a rhesus monkey model for Ebola virus, and it showed broad-spectrum antiviral potential (3). During the 2014–2016 Ebola outbreaks in West Africa, Gilead Sciences provided remdesivir for use in a small number of Ebola-infected patients under a compassionate use protocol (1). However, those results and subsequent trials conducted by the National Institutes of Health showed that remdesivir underperformed compared to other therapeutics (such as monoclonal antibodies), and remdesivir was subsequently abandoned as a treatment option for Ebola virus infections (1).
The Return of Remdesivir
On May 1, 2020, the US Food and Drug Administration announced emergency use authorization (EUA) for “use of remdesivir for the treatment of hospitalized 2019 coronavirus disease (COVID-19) patients.” The foundation for the current research into remdesivir was laid with collaborative studies among Vanderbilt University Medical Center, the University of North Carolina at Chapel Hill and Gilead Sciences (see Investigation of Remdesivir as a Possible Treatment for SARS-CoV-2). Building on earlier research that suggested remdesivir could inhibit RNA replication in both contemporary and emerging coronaviruses (4), the group demonstrated in vivo effectiveness of remdesivir against Middle East respiratory syndrome coronavirus (MERS-CoV) (5).
In a recent study, Pruijssers et al. examined the mechanisms by which remdesivir inhibits SARS-CoV-2 replication in vitro and in vivo (6). Using structural modeling and mutational analysis, the authors studied binding of the active metabolite of remdesivir (RDV-TP) to RdRp. They found that the active site was highly conserved across seven known human SARS-Cov strains as well as other coronaviruses, implying that remdesivir could potentially have broad-spectrum effects.
Next, they performed antiviral assays in several human and monkey cell lines; in one of these—Huh7 (human liver cells)—they used the Nano-Glo® Luciferase Assay System to measure viral replication. The results showed that the relative potency of remdesivir and RDV-TP varied across different cell types. This potency was confirmed in primary human airway epithelial cells. Further, remdesivir did not show cytotoxic effects (using the CellTiter-Glo® Luminescent Cell Viability Assay) in these cells across the range of doses in which potent antiviral effects were observed.
To understand the basis of remdesivir activity in vivo, the researchers constructed a chimeric mouse-adapted SARS-CoV variant encoding the SARS-CoV-2 RdRp. They monitored pulmonary function and viral load in chimeric mice infected with this SARS-CoV variant, following administration of remdesivir or vehicle alone as a control. The data showed dramatic reduction of viral load with remdesivir compared to the control group, as well as significant amelioration of the loss in pulmonary function.
The authors conclude that remdesivir shows therapeutic promise across a broad spectrum of coronavirus infections, due to its interaction with the highly conserved RdRp. Specifically, remdesivir can reduce viral load and improve pulmonary outcomes, supporting its use for patients with COVID-19.
Preliminary results from a randomized clinical trial of remdesivir in patients with severe COVID-19 were reported recently (7). Although remdesivir did not show significant clinical or antiviral effects in this population, further studies with larger patient groups should help provide more data on its effectiveness as a treatment option for COVID-19. Topline results from a Phase 3 clinical trial indicated benefits of remdesivir in hospitalized patients with moderate COVID-19. Gilead Sciences maintains a summary of ongoing remdesivir/COVID-19 clinical trials on its web site.
Interested in tools and resources for viral research? Visit our web page on SARS-CoV-2 Research, Vaccine, and Therapeutic Development.
References
- Development of remdesivir: fact sheet (2020) Gilead Sciences, Inc. [Internet: https://www.gilead.com/-/media/gilead-corporate/files/pdfs/covid-19/gilead_rdv-development-fact-sheet-2020.pdf Accessed: July 24, 2020]
- Yin, W. et al. (2020) Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir. Science 368, 1499–1504.
- Warren, T.K. et al. (2016) Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 431, 381–5.
- Agostoni, M.L. et al. (2018) Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio 9(2), e00221-18.
- Sheahan, T.P. et al. (2020) Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nature Comm. 11, 222.
- Pruijssers, A.J. et al. (2020) Remdesivir inhibits SARS-CoV-2 in human lung cells and chimeric SARS-CoV expressing the SARS-CoV-2 RNA polymerase in mice. Cell Reports 32, 107940.
- Wang, Y. et al. (2020) Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 395, 1569–78.
Related Posts
Latest posts by Ken Doyle (see all)
- Will Artificial Intelligence (AI) Transform the Future of Life Science Research? - February 1, 2024
- RAF Inhibitors: Quantifying Drug-Target Occupancy at Active RAS-RAF Complexes in Live Cells - September 5, 2023
- Synthetic Biology: Minimal Cell, Maximal Opportunity - July 25, 2023
