The phosphotidylinositol 4-kinases (PI 4-kinases) generate phosphotidyl-4-phosphate (PI(4)P) from phosphotylinositol. PI(4)P is an important precursor for other phosphoinositides involved in signaling, such as PI(4,5)P2, which is the substrate of phospholipase C (PLC) and the precursor of DAG and insitol (1,4,5) triphosphate.
There are four different mammalian PI 4-kinases currently described, and these have been divided into two classes based on their sensitivities to wortmannin and adenosine. Type II PI 4-kinases (PI4K2A and PI4K2B) are not sensitive to wortmannin, but are inhibited by the nonspecific inhibitor adenosine; Type III PI 4-kinases (PI4KA and PI4KB) are sensitive to wortmannin.
The functions of the PI 4-kinases and their products are not fully understood. At least one study has shown that PI 4-kinases are important for the proper recycling of synaptic vesicles. The PI 4-kinase from Drosophila, four-wheel drive, is critical for contractile ring formation during cytokinesis. Other studies in yeasts and mammals have shown that PI 4-kinases localize to the Golgi, and in mammals might be critical for proper budding of vesicles from the Golgi. Additionally, type III PI 4-kinases appear to play a role in the replication of hepatitis C virus (HCV) and enteroviruses by participating in the formation of altered host membrane structures. Although, we have hints about their function, to really understand and dissect the precise roles of PI 4-kinases in cells, new tools, such as specific small-molecule inhibitors are required.
Until recently the standard activity assay for PI 4-kinases involved detecting the incorporation of 32P into the PI(4)P product, and then separating the unused 32P from the labeled 32P[PI(4)p] by organic extraction. This assay method, while sensitive, is labor-intensive, produces radioactive and organic waste and is not amenable to high-throughput screening. To find and develop effective inhibitors for this class of molecule a screening assay is needed.
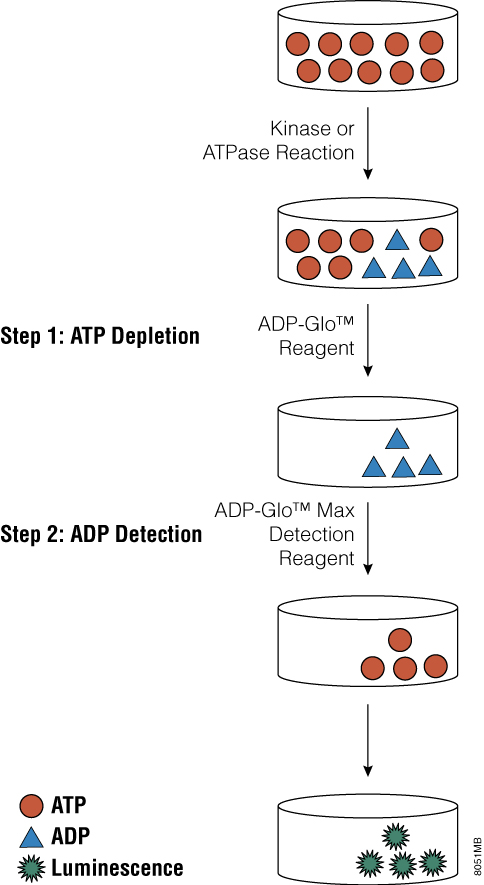
Tai and colleagues recently published a paper in Analytical Biochemistry that describes the use of the ADP-Glo™ Assay Technology to screen for small-molecule inhibitors of PI 4-kinases. ADP-Glo™ Kinase Assay is a luminescent kinase assay that measures ADP formed from a kinase reaction; ADP is converted into ATP, which is converted into light by Ultra-Glo™ Luciferase. The assay is performed in two steps; first, after the kinase reaction, an equal volume of ADP-Glo™ Reagent is added to terminate the kinase reaction and deplete the remaining ATP. In the second step, the Kinase Detection Reagent is added, which simultaneously converts ADP to ATP and allows the newly synthesized ATP to be measured using a coupled luciferase/luciferin reaction. The luminescent signal produced is proportional to the activity of the kinase.
Because the ADP-Glo™ Assay requires purified kinases, the authors first had to express and purify PI4KA and PI4KB. They were unable to do this in E. coli, but they were able to express FLAG-epitope tagged protein in 293T cells. They also expressed a full-length PI4KA protein that contains the D1899A substitution in the kinase catalytic domain, inactivating the kinase activity. They characterized their expressed proteins using the ADP-Glo™ Kinase Assay and saw strong signal-to-background ratios, no signal from their negative controls or the D1899A protein, and no signal in the absence of micelles.
Next they compared data from the ADP-Glo™ Assay with results obtained from the standard isotopic method or results available in the literature. They were able to obtain Km values for PI4KA and PI4KB that corresponded to published values. The wortmannin IC50 values for PIK4A and PIK4B were also comparable to those published in the literature, and dose response curves produced for the small molecular inhibitor PIK93 were similar using the ADP-Glo™ Assay and the standard isotopic assay.
Finally, the authors evaluated the potential of the ADP-Glo™ Assay as a high-throughput screening tool. To assess the assay, they used Z´-factor as their statistical measure, which is a description of the dynamic range and the variability of the assay. Assays with a Z´-factor value greater than 0.5 are typically considered to be robust assays (excellent dynamic range, low variability). In this study, PI4KA activity was measured in a low-volume, 384-well plate assay. One-hundred ninety two wells were treated with wortmannin, 176 with DMSO as a negative control and 16 wells were no-enzyme background controls. In this test the Z´-factor values were 0.72 and 0.74. From these results the authors conclude that the assay would work well for screening in a 384-well system and could likely be optimized for screening in a 1536-well format.
Although the data presented in the paper were with PI4KA and PI4KB, the authors do stated that affinity-purified type III kinases also worked well in the assay and no modifications were required.
The one limitation that they site for the ADP-Glo™ Assay is that it cannot be used with cell lysates or tissues because of its inability to measure PI 4-kinase activity in the presence of other kinases or ATPases. However because the ADP-Glo™ Assay technology does not require radioisotopes or organic extraction and is easily amenable to high-throughput screening, it will be a valuable tool for screening and profiling inhbitors across all four known PI 4-kinases.
More information on kinase biology for drug discovery visit our website.
References
Tai AW, Bojjireddy N, & Balla T (2011). A homogeneous and nonisotopic assay for phosphatidylinositol 4-kinases. Analytical biochemistry, 417 (1), 97-102 PMID: 21704602
Balla, A. and Balla, T. (2006) Phosphatidylinositol 4-kinases: old enzymes with emerging functions. TRENDS in Cell Biology 16, 351–61.
Brill, J. A. (2000) A phospholipid kinase regulates actin organization and intercellular bridge formation during germline cytokinesis. Development 127, 3855–64.
Guo, J. et al. (2003) Phosphatidylinositol 4-kinase type IIa is responsible for the phosphatidylinositol 4-kinase activity associated with synaptic vesicles. Proc. Natl. Acad. Sci. USA 100, 3995–4000.
Zhang, J.H., Chung, T.D. and Oldenburg, K. (1999) A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J. Biomol. Screen. 4, 67–73.