Soon after Amanda Capes-Davis started working with CellBank Australia, she received a request from an exasperated graduate student:
This cell line was handed down to me for my project, but I’m getting strange experimental results with the cells. Can you authenticate the cell line?
After performing genetic analyses, Capes-Davis soon had the answer to the student’s experimental woes: the cells did not come from the human tissue type the student was studying. They weren’t even human—they were mouse cells.
“She’d been given this cell line that was behaving differently than expected, and people thought ‘wow, this is an exciting new variant,’ it could tell her more about a particular disease,” Capes-Davis said. “But no, it was a more sinister reason, unfortunately.”

For Capes-Davis, the founding manager of CellBank Australia, this case of mistaken identity represents just one example of the widespread errors in cell line identity plaguing the scientific community. A more pervasive case is that of MDA-MB-435, a breast cancer cell line established in the 1970s. Starting in the early 2000s, inconsistent results led some to question the authenticity of the cell line. In 2018, researchers with the International Cell Line Authentication Committee (ICLAC) confirmed that MDA-MB-435 is not a line of breast cancer cells from a female donor. It is a melanoma cell line from a male donor, a cell line named M14.
It’s thought that cells from M14 contaminated MDA-MB-435 cultures in the originating laboratory before the cell line was banked in the 1980s. The faster growing cells eventually took over, turning a line made up of breast cancer cells into one made up of melanoma cells. Hundreds of studies since the 1980s using MDA-MB-435 as an in vitro model for breast cancer have been published—hundreds of studies that are now nearly useless.
Cell Line Authentication Protects Research
Avoiding these mistakes is where cell line authentication comes in.
In 2012, the American Type Culture Collection (ATCC) developed a written standard (ASN-0002) recommending that authentication of human cell line identity be adopted as a standard, regular research practice. The document outlined specific recommendations for best authentication practices and methods, specifically recommending a technique called short tandem repeat, or STR, profiling.
STR profiling is a form of genetic analysis that is commonly used in paternity tests and forensic analysis. The procedure can be performed with commercially available kits and equipment and can take as little as three to six hours to generate a profile. Researchers who don’t have the proper equipment can also send their samples out to contract labs for an inexpensive analysis. A July 2021 update to the ATCC document, which Capes-Davis was involved in preparing, outlines how to use online databases and comparison tools to check an experimental STR profile against those of authentic cell lines.
“Because of its connection to the forensics community, it’s a widely available technique, and most labs at an international level would have access to some kind of service if it’s something they’re not able to do themselves,” Capes-Davis said.
“I’m quite serious when I say the laboratory doesn’t really need to understand the technology,” said Doug Storts, Head of Research at Promega and member of ICLAC and the ASN-0002 standard committee. “They just need to understand the consequences of not authenticating their cell lines.”
Those consequences are steep.
There are a range of potential sources for why a cell line is misidentified. In the case of the MDA-MB-435 misidentification, poor cell culture technique likely led to cross-contamination with a more vigorously growing cell line. Whatever the cause, these errors propagate: misidentified cell lines are handed down to new students or are shared with collaborators’ laboratories. Results with these cells then form the basis for research publications and clinical trials, leading to reproducibility issues, a waste of time and resources and retraction of publications. The ICLAC Register of Misidentified Cell Lines lists over 500 existing cell lines that are known to be misidentified.
“People have conducted clinical studies based on results from cell lines that were misidentified,” said Storts. “It’s expensive to perform a clinical study, and then to find out that the data is essentially useless, that’s a problem.”
Since the 2012 ATCC report was published, many journals and funding agencies require authors and researchers to show that they have authenticated the identity of any human cell lines used in their research. But, according to Capes-Davis, good cell line authentication practices are still patchy.
“It’s a bit like pushing an elephant into motion,” said Capes-Davis. “We’ve seen large changes, and yet not much difference in the status quo.”
What is an STR Profile and How Do I Use it?
STRs are elements within the genome where a sequence of 2–7 base pairs is repeated any number of times. These STR segments consistently appear at the same locations, or loci, in the human genome. But the total number of repeat units at a given locus, or allele, can vary between individuals. When you analyze multiple STR loci, the result serves as a “fingerprint” that identifies all cell lines from a particular individual.
In an STR analysis, a standard set of STR loci in a genome are amplified. For example, Promega’s GenePrint® 10 System contains primers for 10 STR loci while the GenePrint® 24 System contains 24. The amplicons are separated by mass and detected using capillary electrophoresis. (Check out this application note for an overview of the cell line authentication process, best practices and methods.)
The resulting profile will show which alleles are present at each STR locus within the cell line, displayed in a tabular format where each repeat unit is represented by a number. This profile can be compared with reference profiles to examine how closely the profiles match or can be searched against a database of STR profiles.
Generally, for two STR profiles to be considered a “match” for cell line authentication, they need to be at least 80% similar. That value accounts for inevitable genetic drift over time for cultured cells. To balance effects of genetic drift with the need for increased accuracy in profile matching, the recommended number of STR loci used for cell line authentication was increased in the 2021 ATCC document from 8 to 13. That change brings the probability of obtaining a match for unrelated cell lines down from 1 in 10-8 to 3 in 10-15, a sevenfold change.
The team responsible for updating the ATCC guidelines determined that the increase in the number of STR loci was required to help distinguish cell lines with genetic instability. For example, the Jurkat leukemia cell line is fast-growing and a common contaminant of other cell lines. A high level of microsatellite instability in this cell line, however, makes it challenging to identify Jurkat cells as a contaminant if only eight STR loci are analyzed.
How Can You Preserve Your Cell Line’s Integrity?
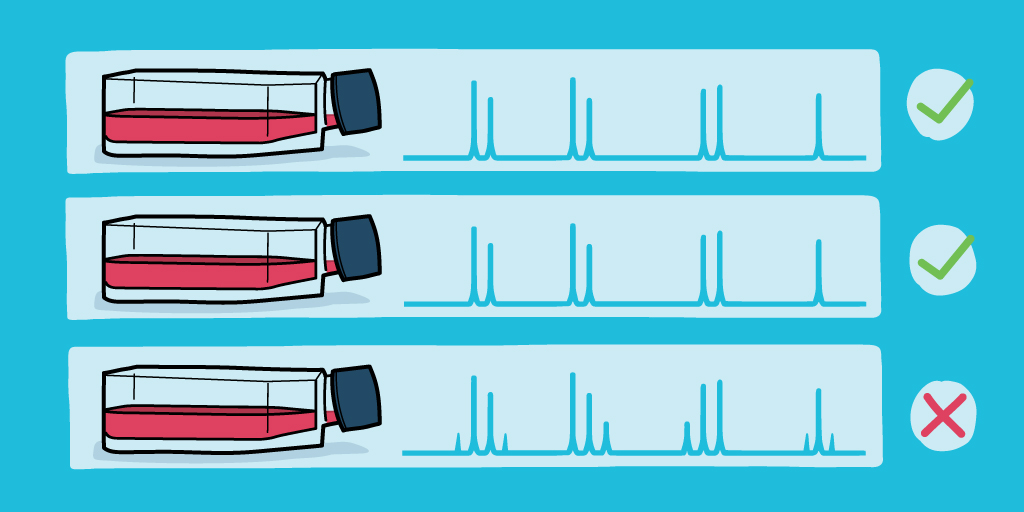
If you’re working with cell lines, cell line authentication needs to be a standard part of your cell culture toolbox. If your lab has the equipment, then learn how to do the analysis. If it doesn’t, find a reputable lab that can do the analysis for you. You can also check out databases that list commonly misidentified cell lines to see if the cell line you’re working with is known to be problematic.
Most importantly, cell line authentication isn’t a “one and done” practice. Cell lines need to be authenticated regularly to catch cross-contamination early. The instances where you should authenticate your cells lines are:
- at the beginning of a project
- at regular intervals during a project
- when receiving or establishing a new cell line
- after routine cell line passaging
- when freezing stock lines
- when observing inconsistent results
- when preparing to publish
Want to learn more about cell line authentication and STR profiling? Check out the Check out the Cell Line Authentication Resources page.
Latest posts by Jordan Nutting (see all)
- Silencing the Immunogenicity of AAV Vectors - April 4, 2024
- Discovering Cyclic Peptides with a “One-Pot” Synthesis and Screening Method - February 29, 2024
- How do Self-Amplifying RNA Vaccines Work? - February 6, 2024
