Advantages of Spheroids
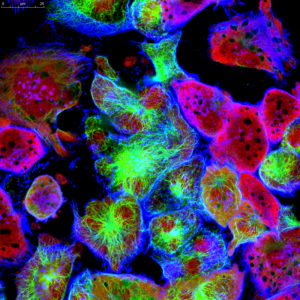
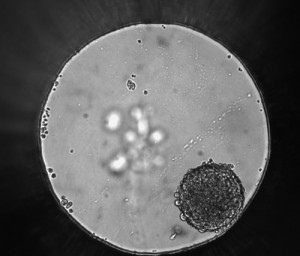
In the past decade, there has been a sharp rise in studies using spheroids as cell models for basic research and drug discovery. Spheroids are self-organized aggregation of cells that form a spherical mass, and they have become widely popular because they are much more physiologically relevant compared to flat 2D cell cultures.
In spheroids, the inner cells have less access to nutrients and oxygen compared to the outer layer, forming a natural gradient. As a result, metabolite concentration and cellular state such as proliferation and differentiation, can be very different at the periphery compared to the inner core. This phenomenon, known as “heterogeneity”, makes 3D tumor spheroids much more representative of actual tumors in the human body.
Continue reading “MISpheroID: A Knowledgebase to Improve Reproducibility in Spheroid Research”