In today’s post, guest blogger, Martha O’Brien, PhD, provides a preview of her upcoming AAI poster and block symposium talk on the inflammasome, caspase-1 activity and pyroptosis.
Responding rapidly to microbial pathogens and damage-associated molecular markers is critical to our innate immune system. Caspase-1 is pivotal in this process leading to processing and release of essential cytokines and an immunogenic form of cell death, termed pyroptosis. Upon sensing pathogen-associated and damage-associated molecular patterns (PAMPs and DAMPs), innate immune cells form inflammasome protein complexes that recruit and activate caspase-1 (canonical inflammasomes). In addition, other inflammatory caspases, 4 and 5 in humans and 11 in mice, directly bind bacterial lipopolysaccharides (LPS), triggering pyroptosis (non-canonical inflammasome). LPS-triggered non-canonical inflammasomes in mice and humans ultimately lead to canonical inflammasome engagement and caspase-1 activation (1–3). Caspase-1 was originally termed interleukin converting enzyme (ICE) for its well-established role in processing IL-1ß and IL-18, two important inflammation cytokines. How caspase-1 mediates pyroptosis is less well understood, but is beginning to be delineated. Recently, a substrate of the inflammatory caspases, gasdermin D, was identified and its processed fragment, gasdermin-N domain, was shown to be required for pyroptosis in non-canonical inflammasome circumstances (4, 5). The precise role of gasdermin D in canonical inflammasome-triggered pyroptosis is still under investigation. Linking inflammatory caspases directly to pyroptosis is a notable step in understanding the mechanism of this important form of cell death.
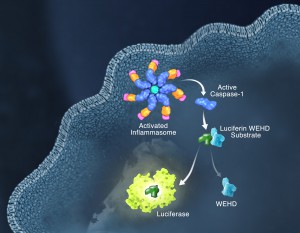
Pyroptosis is clearly one means of releasing processed IL-1ß and IL-18 from the cell. However depending on the cell type and stimulus, there is evidence for inflammasome engagement, caspase-1 activation, and release of IL-1ß in the absence of cell death (6, 7). On the flip-side there is also evidence for caspase-1 mediated pyroptosis that helps clear bacteria, independent of IL-1ß and IL-18 involvement (8). To enable further studies on the inflammasome and in particular, assessing the connections between caspase-1 activation, pyroptosis, and cytokine release, Promega developed a new tool to conveniently monitor caspase-1 activation, the Caspase-Glo® 1 Inflammasome Assay. This bioluminescent, plate-based assay is used to measure caspase-1 activity directly in cell cultures or to monitor released caspase-1 activity in culture medium from treated cells. This flexibility allows easy multiplexing to monitor all three outcomes of inflammasome stimulation; caspase-1 activity, pyroptosis, and release of IL-1ß and IL-18. Caspase-1 activation typically is monitored indirectly with western blots of processed caspase-1. Now the activity of the enzyme can be monitored directly, providing accurate information on temporal aspects of the inflammasome. The assay can be readily combined with real-time measures of cell death (e.g., CellTox™ Green Cytotoxicity Assay) and some of the culture medium can be removed for IL-1ß/IL-18 assessment, leaving the cells and remaining culture medium for caspase-1 activity measurements.
References
- Schmid-Burgk et al. (2015) Caspase-4 mediates non-canonical activation of the NLRP3 inflammasome in human myeloid cells. J. Immunol. 45, 2911–7.
- Baker et al. (2015) NLRP3 inflammasome activation downstream of cytoplasmic LPS recognition by both caspase-4 and caspase-5. J. Immunol. 45, 2918–26.
- Ruhl, S. and P. Broz (2015) Caspase-11 activates a canonical NLRP3 inflammasome by promoting K+ Eur. J. Immunol. 45, 2927–36.
- Shi et al. (2015) Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–5.
- Kayagaki et al. (2015) Caspase-11 cleaves gasdermin D for non-canonical inflammasome signaling. Nature 526, 666–71.
- Gaidt et al. (2016) Human monocytes engage an alternative inflammasome pathway. Immunity 44, 833–46.
- Chen et al. (2014) The neutrophil NLRC4 inflammasome selectively promotes IL-1ß maturation without pyroptosis during acute. Salmonella Cell Reports 8, 570–82.
- Miao et al. (2010) Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nature Immunology 11, 1136–42.