Live animal in vivo imaging is a common and useful tool for research, but current tools could be better. Two recent papers discuss adaptations of BRET technology combining the brightness of fluorescence with the low background of a bioluminescence reaction to create enhanced in vivo imaging capabilities.
The key is to image photons at wavelengths above 600nm, as lower wavelengths are absorbed by heme-containing proteins (Chu, J., et al., 2016 ). Fluorescent protein use in vivo is limited because the proteins must be excited by an external light source, which generates autofluorescence and has limited penetration due to absorption by tissues. Bioluminescence imaging continues to be a solution, especially firefly luciferase (612nm emission at 37°C), but its use typically requires long image acquisition times. Other luciferases, like NanoLuc, Renilla, and Gaussia, etc. either do not produce enough light or the wavelengths are readily absorbed by tissues, limiting their use to near-surface imaging.
The two papers discussed here illustrate how researchers have combined NanoLuc® luciferase with a fluorescent protein to harness bioluminescent resonance energy transfer (BRET) for brighter in vivo imaging reporters.
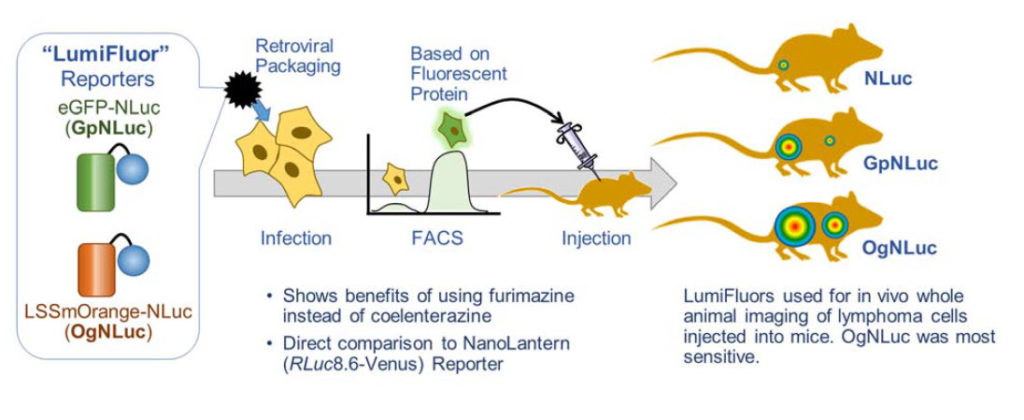
LumiFluor In vivo Imaging Reporters
Schaub, F.X., et al. (2015) Fluorophore-NanoLuc BRET reporters enable sensitive in vivo optical imaging and flow cytometry for monitoring tumorigenesis. Cancer Res. 75, 5023-33. PubMed ID: 26424696
This paper lays out the reasons why a BRET reporter can greatly improve deep tissue imaging. The lab made two potential BRET reporters with NanoLuc® Luciferase fused to enhanced green fluorescent protein (GpNluc) and a large Stokes shift orange fluorescent protein, LSSmOrange (OgNLuc). The lab has dubbed these LumiFluor Reporters. Both fluorescent proteins could be excited by the NanoLuc emission. The constructs were designed to be packaged into a retrovirus for infection of host cells. The fluorescent moiety of the BRET reporter was used to sort successfully infected/expressing cells by flow cytometry. The isolated cells were injected into mice and imaged after i.p. or i.v. administration of the furimazine substrate for NanoLuc luciferase. Both GpNLuc and OgNLuc BRET reporters produced bright signals, with OgNLuc being the brightest since it had an emission near 600nm. The constructs for GpNLuc and OgNLuc are available through Addgene (Cat.# 70185 and 70186, respectively).
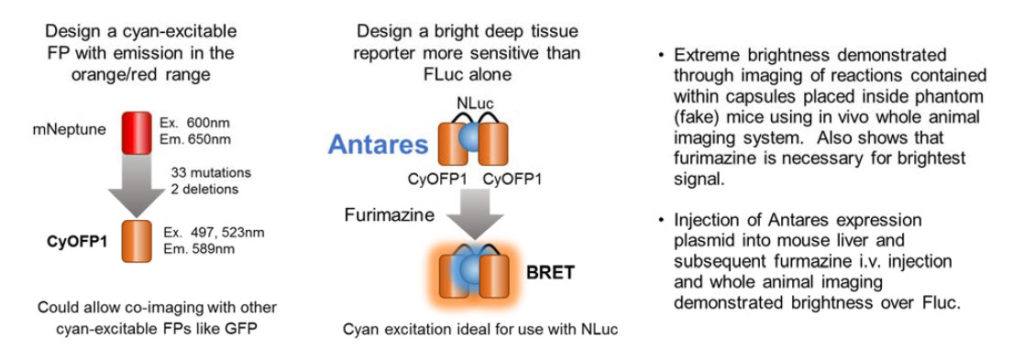
Antares In vivo Imaging Reporter
Chu, J., et al. (2016) A bright cyan-excitable orange fluorescent protein facilitates dual-emission microscopy and enhances bioluminescence imaging in vivo. Nature Biotechnol. 34, 760-7. PubMed ID: 27240196
These authors set out to design a better fluorescent protein that could be co-imaged with GFP using the same wavelength for excitation. The result was the cyan-excitable orange fluorescent protein, CyOFP1. CyOFP1 was molecularly evolved from mNeptune2 through 33 mutations and 2 deletions. CyOFP1 had a higher quantum yield, brightness and improved maturation time compared to similar orange fluorescent proteins. The authors note how CyOFP1 could be excited by NanoLuc® luciferase (NLuc) and set about to create an in vivo imaging reporter by linking two CyOFP1 molecules to NLuc (CyOFP1-NLuc-CyOFP1; Antares). Tests of brightness were made with a variety of in vivo imaging reporters including firefly and Renilla luciferase-based BRET in vivo imaging reporters, and Antares proved extremely bright. Direct injection of plasmids for Antares or firefly luciferase into mouse livers followed by i.v. injection of furimazine or luciferin 24 hours later demonstrated a more than 2-fold brighter signal in this model. The construct for Antares is available through Addgene (Cat. # 74729).
Pairing NanoLuc® luciferase with fluorescent proteins has allowed researchers to make innovative tools not possible with other luciferases. These papers shift the bright blue luminescence of NanoLuc luciferase to more imaging-friendly wavelengths thus gaining the brightness of a fluorescent protein with the low background of luminescence. Both groups deposited clones in Addgene (www.addgene.org), a non-profit plasmid repository. Researchers around the world can obtain these tools for a small fee and use them in their lab immediately.
For help answering questions about NanoLuc® luciferase or BRET, contact a Promega Technical Services Scientist.
